Abstract
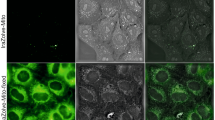
In the search for new biological imaging agents, metal coordination compounds able to emit from triplet metal-to-ligand charge transfer (MLCT) states offer many advantages as luminescent probes of DNA structure. However, poor cellular uptake restricts their use in live cells. Here, we present a dinuclear ruthenium(II) polypyridyl system that works as a multifunctional biological imaging agent staining the DNA of eukaryotic and prokaryotic cells for both luminescence and transition electron microscopy. This MLCT ‘light switch’ complex directly images nuclear DNA of living cells without requiring prior membrane permeabilization. Furthermore, inhibition and transmission electron microscopy studies show this to be via a non-endocytotic, but temperature-dependent, mechanism of cellular uptake in MCF-7 cells, and confocal microscopy reveals multiple emission peaks that function as markers for cellular DNA structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tsien, R. Y., Ernst, L. & Waggoner, A. in Handbook of Biological Confocal Microscopy 3rd edn (ed. J. B. Pawley) 338–352 (Springer, 2006).
Martin, R. M., Leonhardt, H. & Cardoso, M. C. DNA labeling in living cells. Cytometry Part A 67, 45–52 (2005).
Pfeifer, G. P., You, Y.-H. & Besaratinia, A. Mutations induced by ultraviolet light. Mutat. Res. 571, 19–31 (2005).
Pandya, S., Yu, J. & Parker, D. Engineering emissive europium and terbium complexes for molecular imaging and sensing. Dalton Trans. 2757–2766 (2006).
Botchway, S. W. et al. Time-resolved and two-photon emission imaging microscopy of live cells with inert platinum complexes. Proc. Natl Acad. Sci. USA 105, 16071–16076 (2008).
Friedman, A. E., Chambron, J. C., Sauvage, J. P., Turro, N. J. & Barton, J. K. A molecular light switch for DNA: Ru(bpy)2(dppz)2+. J. Am. Chem. Soc. 112, 4960–4962 (1990).
Zeglis, B. M., Pierre, V. C. & Barton, J. K. Metallo-intercalators and metallo-insertors. Chem. Commun. 4565–4579 (2007).
Metcalfe, C. & Thomas, J. A. Kinetically inert transition metal complexes that reversibly bind to DNA. Chem. Soc. Rev. 32, 215–224 (2003).
Jiménez-Hernández, M. E., Orellana, G., Montero, F. & Portolés, M. T. A ruthenium probe for cell viability measurement using flow cytometry, confocal microscopy and time-resolved luminescence. Photochem. Photobiol. 72, 28–34 (2000).
Onfelt, B., Gostring, L., Lincoln, P., Norden, B. & Onfelt, A. Cell studies of the bisintercalator [u-C4(cpdppz)2-(phen)4Ru2]4+ : toxic effects and properties as a light emitting DNA probe in V79 Chinese hampster cells. Mutagenesis 17, 317–320 (2002).
Amoroso, A. J. et al. Rhenium fac tricarbonyl bisimine complexes: biologically useful fluorochromes for cell imaging applications. Chem. Commun. 3066–3068 (2007).
Puckett, C. A. & Barton, J. K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 129, 46–47 (2007).
Puckett, C. A. & Barton, J. K. Mechanism of cellular uptake of a ruthenium polypyridyl complex. Biochemistry 47, 11711–11716 (2008).
Lo, K. K. W., Lee, T. K. M., Lau, J. S. Y., Poon, W. L. & Cheng, S. H. Luminescent biological probes derived from ruthenium(II) estradiol polypyridine complexes. Inorg. Chem. 47, 200–208 (2008).
Neugebauer, U. et al. Ruthenium polypyridyl peptide conjugates: membrane permeable probes for cellular imaging. Chem. Commun. 5307–5309 (2008).
Bolger, J., Gourdon, A., Ishow, E. & Launay, J.-P. Mononuclear and binuclear tetrapyrido [3.2-a: 2′, 3′-c: 3″, 2″-h: 2″′, 3″′-j] phenazine (tpphz) ruthenium and osmium complexes. Inorg. Chem. 35, 2937–2944 (1996).
Rajput, C., Rutkaite, R., Swanson, L., Haq, I. & Thomas, J. A. Dinuclear monointercalating Ru(II) complexes that display high affinity binding to duplex and quadruplex DNA. Chem. Eur. J. 12, 4611–4619 (2006).
Lutterman, D. A. et al. Intercalation is not required for DNA light-switch behavior. J. Am. Chem. Soc. 130, 1163–1170 (2008).
Campagna, S., Serroni, S., Bodige, S. & MacDonnell, F. M. Absorption spectra, photophysical properties, and redox behavior of stereochemically pure dendritic ruthenium(II) tetramers and related dinuclear and mononuclear complexes. Inorg. Chem. 38, 692–701 (1999).
Mailaender, C. et al. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150, 853–864 (2004).
Ziegler, H. K. & Unanue, E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc. Natl Acad. Sci. USA 79, 175–178 (1982).
Wang, L. H., Rothberg, K. G. & Anderson, R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 (1993).
Schnitzer, J. E., Oh, P., Pinney, E. & Allard, J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232 (1994).
Elkjaer, M. L., Birn, H., Agre, P., Christensen, E. I. & Nielsen, S. Effects of microtubule disruption on endocytosis, membrane recycling and polarized distribution of Aquaporin-1 and gp330 in proximal tubule cells. Eur. J. Cell Biol. 67, 57–72 (1995).
Musatkina, E., Amouri, H., Lamoureux, M., Chepurnykh, T. & Cordier, C. Mono- and dicarboxylic polypyridyl-Ru complexes as potential cell DNA dyes and transfection agents. J. Inorg. Biochem. 101, 1086–1089 (2007).
Brunner, J. & Barton, J. K. Targeting DNA Mismatches with rhodium intercalators functionalized with a cell-penetrating peptide. Biochemistry 45, 12295–12302 (2006).
Hurley, L. H. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2, 188–200 (2002).
Douglas, M. P. & Rogers, S. O. DNA damage caused by common cytological fixatives. Mutat. Res. 401, 77–88 (1998).
Canela, A., Vera, E., Klatt, P. & Blasco, M. A. High-throughput telomere length quantification by FISH and its application to human population studies. Proc. Natl Acad. Sci. USA 104, 5300–5305 (2007).
Acknowledgements
The author thanks C. Hill for TEM assistance and E. Smythe for helpful discussions. This work was supported by the EPSRC (UK) and the White Rose LSI-DTC.
Author information
Authors and Affiliations
Contributions
M.R.G., G.B. and J.A.T. conceived and designed the experiments, M.R.G. and J.G.-L. performed the experiments, all the authors analysed the data, G.B., S.F., C.S. and J.A.T. contributed materials and analysis tools, M.R.G., G.B. and J.A.T. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1123 kb)
Rights and permissions
About this article
Cite this article
Gill, M., Garcia-Lara, J., Foster, S. et al. A ruthenium(II) polypyridyl complex for direct imaging of DNA structure in living cells. Nature Chem 1, 662–667 (2009). https://doi.org/10.1038/nchem.406
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.406
This article is cited by
-
Fast detection, a precise and sensitive diagnostic agent for breast cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
A Biophysical Study of Ru(II) Polypyridyl Complex, Properties and its Interaction with DNA
Journal of Fluorescence (2022)
-
Ultrafast excited state dynamics and light-switching of [Ru(phen)2(dppz)]2+ in G-quadruplex DNA
Communications Chemistry (2021)
-
Recent advances in cancer photo-theranostics: the synergistic combination of transition metal complexes and gold nanostructures
SN Applied Sciences (2021)
-
Red-emitting fluorescence probe for sensing viscosity in living cells
Chemical Papers (2020)