Abstract
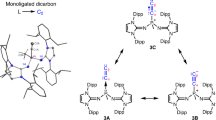
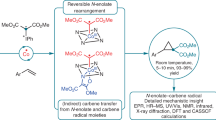
Bonds are at the very heart of chemistry. Although the order of carbon–carbon bonds only extends to triple bonds, metal–metal bond orders of up to five are known for stable compounds, particularly between chromium atoms. Carbometallation and especially carboalumination reactions of carbon–carbon double and triple bonds are a well established synthetic protocol in organometallic chemistry and organic synthesis. We now extend these reactions to compounds containing chromium–chromium quintuple bonds. Analogous reactivity patterns indicate that such quintuple bonds are not as exotic as previously assumed. Yet the particularities of these reactions reflect the specific nature of the high metal–metal bond orders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pauling, L. The Nature of the Chemical Bond 3rd edn (Wiley-VCH, 1973).
Lewis, G. N. The atom and the molecule. J. Am. Chem. Soc. 38, 762–785 (1916).
Frenking, G. & Shaik, S. 90 years of chemical bonding. J. Comput. Chem. 28, 1–3 (2007).
Shaik, S. The Lewis legacy: The chemical bond—a territory and heartland of chemistry. J. Comput. Chem. 28, 51–61 (2007).
Nguyen, T. et al. Synthesis of a stable compound with fivefold bonding between two chromium(i) centers. Science 310, 844–847 (2005).
Wolf, R. et al. Substituent effects in formally quintuple-bonded ArCrCrAr compounds (Ar = terphenyl) and related species. Inorg. Chem. 46, 11277–11290 (2007).
Kreisel, K. A., Yap, G. P. A., Dmitrenko, O., Landis, C. R. & Theopold, K. H. The shortest metal–metal bond yet: molecular and electronic structure of a dinuclear chromium diazadiene complex. J. Am. Chem. Soc. 129, 14162–14163 (2007).
Tsai, Y.-C. et al. Remarkably short metal–metal bonds: a lantern-type quintuply bonded dichromium(i) complex. Angew. Chem. Int. Ed. 47, 7250–7253 (2008).
Noor, A., Wagner, F. R. & Kempe, R. Metal–metal distances at the limit: A coordination compound with an ultrashort chromium–chromium bond. Angew. Chem. Int. Ed. 47, 7246–7249 (2008).
Hsu, C.-W. et al. Quintuply-bonded dichromium(i) complexes featuring metal–metal bond lengths of 1.74 Å. Angew. Chem. Int. Ed. 47, 9933–9936 (2008).
Gagliardi, L. & Ross, B. O. Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond. Nature 433, 848–851 (2005).
Roos, B. O., Borin, A. C. & Gagliargi, L. Reaching the maximum multiplicity of the covalent chemical bond. Angew. Chem. Int. Ed. 46, 1469–1472 (2007).
Frenking, G. & Tonner, R. Theoretical chemistry: The six-bond bound. Nature 446, 276–277 (2007).
Merino, G., Donald, K. J., D'Acchioli, J. S. & Hoffmann, R. The many ways to have a quintuple bond. J. Am. Chem. Soc. 129, 15295–15302 (2007).
Radius, U. & Breher, F. To boldly pass the metal–metal quadruple bond. Angew. Chem. Int. Ed. 45, 3006–3010 (2006).
Frenking, G. Building a quintuple bond. Science 310, 796–797 (2005).
Brynda, M., Gagliardi, L., Widmark, P.-O., Power, P. P. & Roos, B. O. A quantum chemical study of the quintuple bond between two chromium centers in [PhCrCrPh]: Trans-bent versus linear geometry. Angew. Chem. Int. Ed. 45, 3804–3807 (2006).
La Macchia, G., Brynda, M. & Gagliardi, L. Quantum chemical calculations predict the diphenyl diuranium compound [PhUUPh] to have a stable 1Ag ground state. Angew. Chem. Int. Ed. 45, 6210–6213 (2006).
Huang, J., Li, Q., Ren, H., Su, H, & Yang, J. Single quintuple bond [PhCrCrPh] molecule as a possible molecular switch. J. Chem. Phys. 125, 184713 (2006).
Roos, B. O., Malmqvist, P.-A. & Gagliardi, L. Exploring the actinide–actinide bond: Theoretical studies of the chemical bond in Ac2, Th2, Pa2, and U2 . J. Am. Chem. Soc. 128, 17000–17006 (2006).
Weinhold, F. & Landis, C. R. High bond orders in metal–metal bonding. Science 316, 61–63 (2007).
La Macchia, G., Gagliardi, L., Power, P. P. & Brynda, M. Large differences in secondary metal–arene interactions in the transition-metal dimers ArMMAr (Ar = terphenyl; M = Cr, Fe, or Co): Implications for Cr–Cr quintuple bonding. J. Am. Chem. Soc. 130, 5104–5114 (2008).
La Macchia, G., Aquilante, F., Veryazov, V., Ross, B. O. & Gagliardi, L. Bond length and bond order in one of the shortest Cr–Cr bonds. Inorg. Chem. 47, 11455–11457 (2008).
Bondybey, V. E. & English, J. H. Electronic structure and vibrational frequency of diatomic chromium (Cr2). Chem. Phys. Lett. 94, 443–447 (1983).
Cotton, F. A., Murillo, L. A. & Walton, R. A. Multiple Bonds Between Metal Atoms 3rd edn (Springer, 2005).
Gade, L. H. Coordination Chemistry (Wiley-VCH, 1998).
Hein, F. & Tille, D. Zur existenz substituierter chromphenylverbindungen. Z. Anorg. Allg. Chem. 329, 72–82 (1964).
Cotton, F. A. & Koch, S. A. Rational preparation and structural study of a dichromium o-oxophenyl compound: The shortest metal-to-metal bond yet observed. Inorg. Chem. 17, 2021–2024 (1978).
Cotton, F. A., Koch, S. A. & Millar, M. Tetrakis(2-methoxy-5-methylphenyl) dichromium. Inorg. Chem. 17, 2084–2086 (1978).
Horvath, S., Gorelsky, S. I., Gambarotta, S. & Korobkov, I. Breaking the 1.80 Å barrier of the Cr–Cr multiple bond between Cr(ii) atoms. Angew. Chem. Int. Ed. 47, 9937–9940 (2008).
Elschenbroich, Ch. Organometallchemie 5th edn (Teubner, 2005).
Fallis, A. G. & Forgione, P. Metal mediated carbometallation of alkynes and alkenes containing adjacent heteroaoms Tetrahedron 57, 5899–5913 (2001).
Nishino, H., Kochi, J. K. Unusually stable chromium(v) perfluoropinacolate. Inorg. Chim. Acta 174, 93–102 (1990).
Danopoulos, A. A., Wilkinson, G., Sweet, T. K. N. & Hursthouse, M. B. Reactions of bis(mesitylimido)bis(arylthiolato)chromium(vi) compounds. Polyhedron 15, 873–879 (1996).
Herberhold, M., Kremnitz, W., Razavi, A., Schollhorn, H. & Thewalt, U. [(η5-C5Me5)CrO2]2—a binuclear oxo complex of chromium(v). Angew. Chem. Int. Ed. 24, 601–602 (1985).
Tsai, Y. C., Wang, P.-Y., Chen, S.-A. & Chen, J.-M. Inverted-sandwich dichromium(i) complexes supported by two β-diketiminates: A multielectron reductant and syntheses of chromium dioxo and imido. J. Am. Chem. Soc. 129, 8066–8067 (2007).
Monillas, W. H., Yap, G. P. A., MacAdams, L. A. & Theopold, K. H. Binding and activation of small molecules by three-coordinate Cr(i). J. Am. Chem. Soc. 129, 8090–8091 (2007).
Yu, Q., Purath, A., Donchev, A. & Schnöckel, H. The first structurally characterized coordination compound containing a direct Al–Cr bonding: Cp*Al-Cr(CO)5 . J. Organomet. Chem. 584, 94–97 (1999).
Folsing, H. et al. Synthesis and structure of adduct stabilized Group III metal transition metal carbonyl complexes: new examples for Fe–Ga, Fe–In, W–Al, Cr–Al and Cr–Ga bonds. J. Organomet. Chem. 606, 132–140 (2000).
Kreisel, K. A., Yap, G. P. A. & Theopold, K. H. A bimetallic N-heterocyclic carbene complex featuring a short Cr–Cr distance. Chem. Commun. 1510–1511 (2007).
Wei, P. & Stephan D. W. Cationic and neutral phosphido-bridged pentamethylcyclopentadienyl-chromium dimers. Organometallics 22, 1712–1717 (2003).
Heintz, R. A., Ostrander, R. L., Rheingold, A. L. & Theopold K. H. [(η5-C5R5)CrR]n: A new class of paramagnetic alkyls and hydrides of divalent chromium. J. Am. Chem. Soc. 116, 11387–11396 (1994).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 38, 3098–3100 (1988).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990).
Reed, A. E., Curtiss, L. A. & Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Wiberg, K. B. Application of the Pople–Santry–Segal complete neglect of differential overlap method to the cyclopropyl-carbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968).
Acknowledgements
We thank C. Döring for his support in the X-ray lab.
Author information
Authors and Affiliations
Contributions
A.N. carried out the synthesis experiments and analysed the spectroscopic data. G.G. performed the X-ray single crystal structure analyses. R.M. and M.K. carried out the computational studies. S.D. performed the magnetic susceptibility experiments. R.K. originated the central idea and wrote the manuscript with contributions from all the co-authors.
Corresponding authors
Supplementary information
Supplementary information
Supplementary information (PDF 833 kb)
Supplementary information
Crystallographic information for compound 2 (CIF 15 kb)
Supplementary information
Crystallographic information for compound 3 (CIF 15 kb)
Supplementary information
Crystallographic information for compound 4 (CIF 14 kb)
Supplementary information
Crystallographic information for compound 5 (CIF 32 kb)
Rights and permissions
About this article
Cite this article
Noor, A., Glatz, G., Müller, R. et al. Carboalumination of a chromium–chromium quintuple bond. Nature Chem 1, 322–325 (2009). https://doi.org/10.1038/nchem.255
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.255
This article is cited by
-
Ultrashort metal–metal distances and extreme bond orders
Nature Chemistry (2009)