Abstract
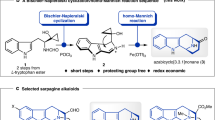
Cascade reactions enable the rapid build-up of molecular complexity from relatively simple starting materials. Both rapid construction and the ability to prepare related structures are crucial to the study of biological activities. Here, we report an efficient, highly enantioselective and diastereoselective total synthesis of ricciocarpin A. The key feature of the synthesis is a one-pot, three-step, organocatalytic reductive Michael–Tishchenko cascade. The conciseness and flexibility of this approach not only resulted in the synthesis of the natural product, but also of its antipode and four other structural analogues. A preliminary biological evaluation of these compounds identified an analogue with significantly improved molluscicidal activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mukherjee, S., Yang, J. W., Hoffmann, S. & List, B. Asymmetric enamine catalysis. Chem. Rev. 107, 5471–5569 (2007).
Erkkilä, A., Majander, I. & Pihko, P. M. Iminium catalysis. Chem. Rev. 107, 5416–5470 (2007).
List, B. Asymmetric aminocatalysis. Synlett 11, 1675–1686 (2001).
Notz, W., Tanaka, F. & Barbas, C. F. III . Enamine-based oganocatalysis with proline and diamines: the development of direct catalytic asymmetric aldol, Mannich, Michael, and Diels–Alder reactions. Acc. Chem. Res. 37, 580–591 (2004).
Lelais, G. & MacMillan, D. W. C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichim. Acta 39, 79–87 (2006).
Yang, J. W., Hechavarria Fonseca, M. T. & List, B. Catalytic asymmetric reductive Michael cyclization. J. Am. Chem. Soc. 127, 15036–15037 (2005).
Hechavarria Fonseca, M. T. & List, B. Catalytic asymmetric intramolecular Michael reaction of aldehydes. Angew. Chem. Int. Ed. 43, 3958–3960 (2004).
Halland, N., Hazell, R. G. & Jørgensen, K. A. Organocatalytic asymmetric conjugate addition of nitroalkanes to α,β-unsaturated enones using novel imidazoline catalysts. J. Org. Chem. 67, 8331–8338 (2002).
Peelen, T. J., Chi, Y. & Gellman, S. H. Enantioselective organocatalytic Michael additions of aldehydes to enones with imidazolidinones: cocatalyst effects and evidence for an enamine intermediate. J. Am. Chem. Soc., 127, 11598–11599 (2005).
Hayashi, Y., Gotoh, H., Tamura, T., Yamaguchi, H., Masui, R. & Shoji, M. Cysteine-derived organocatalyst in a highly enantioselective intramolecular Michael reaction. J. Am. Chem. Soc. 127, 16028–16029 (2005).
Mase, N., Watanabe, K., Yoda, H., Takabe, K., Tanaka, F. & Barbas, C. F. III. Organocatalytic direct Michael reaction of ketones and aldehydes with β-nitrostyrene in brine. J. Am. Chem. Soc. 128, 4966–4967 (2006).
Weinstain, R., Lerner, R. A., Barbas, C. F. III & Shabat, D. Antibody-catalyzed asymmetric intramolecular Michael addition of aldehydes and ketones to yield the disfavored cis-product. J. Am. Chem. Soc. 127, 13104–13105 (2005).
Yong, H., Walji, A. M., Larsen, C. H. & MacMillan, D. W. C. Enantioselective organo-cascade catalysis. J. Am. Chem. Soc. 127, 15051–15053 (2005).
Wurzel, G. & Becker, H. Sesquiterpenoids from the liverwort Ricciocarpos natans. Phytochemistry 29, 2565–2568 (1990).
Wurzel, G., Becker, H., Eicher, H. T. & Tiefensee, K. Molluscicidal properties of constituents from the liverwort Ricciocarpos natans and of synthetic lunularic acid derivatives. Planta Med. 56, 444–445 (1990).
Zinsmeister, H. D., Becker, H. & Eicher, T. Bryophytes, a source of biologically active, naturally occurring material? Angew. Chem. Int. Ed. 30, 130–147 (1991).
Schreier, T. M., Dawson, V. K., Choi, Y., Spanjers, N. J. & Boogaard, M. A. J. Determination of niclosamide residues in rainbow trout (Oncorhynchus mykiss) and channel catfish (Ictalurus punctatus) fillet tissue by high-performance liquid chromatography. Agric. Food Chem. 48, 2212–2215 (2000).
Eicher, T., Massonne, K. & Herrmann, M. Synthese von bryophyten-inhaltsstoffen 4. Synthesen des Ricciocarpins A. Synthesis 1173–1176 (1991).
Ihara, M., Suzuki, S., Taniguchi, N. & Fukumoto, K. Deconjugation of α,β-unsaturated esters and an intramolecular Michael reaction of bis-α,β-unsaturated esters with trialkylsilyl trifluoromethanesulfonate in the presence of tertiary amine: synthesis of (±)-ricciocarpin A. J. Chem. Soc. Perkin Trans. 1 2251–2258 (1993).
Ihara, M., Suzuki, S., Taniguchi, N. & Fukumoto, K. Intramolecular Michael reaction using trialkylsilyl trifluoromethanesulfonates and tertiary amine system: total synthesis of (±)-ricciocarpin A. J. Chem. Soc. Chem. Commun. 755–756 (1993).
Takeda, K., Ohkawa, N., Hori, K., Koizumi, T. & Yoshii, E. A short synthesis of (±)-ricciocarpin A using intramolecular reductive Michael reaction. Heterocycles 47, 277–282 (1998).
Agapiou, K. & Krische, M. J. Catalytic crossed Michael cycloisomerization of thioenoates: total synthesis of (±)-ricciocarpin A. Org. Lett. 5, 1737–1740 (2003).
Held, C., Frohlich, R. & Metz, P. Enantioselective synthesis of the ricciocarpins A and B. Angew. Chem. Int. Ed. 40, 1058–1060 (2001).
Held, C., Frohlich, R. & Metz, P. Enantioselective synthesis of the molluscicidal furanosesquiterpene lactones ricciocarpin A and ricciocarpin B via ring closing metathesis. Adv. Synth. Catal. 344, 720–727 (2002).
Sibi, M. P. & He, L. Application of enantioselective radical reactions: synthesis of (+)-ricciocarpins A and B. Org. Lett. 6, 1749–1752 (2004).
Ning-Wei, J. & Hsing-Jang, L. An enantioselective total synthesis of (+)-ricciocarpin A. Org. Lett. 8, 151–153 (2006).
Palombo, E., Audran, G. & Montir, H. Enantioselective synthesis of (+)-ricciocarpin A using an auxiliary hydroxyl group and a diastereofacial selectivity based methodology. Synlett 13, 2104–2106 (2005).
Connon, S. J. & Blechert, S. Recent developments in olefin cross-metathesis. Angew. Chem. Int. Ed. 42, 1900–1923 (2003).
Grela, K., Harutyunyan, S. & Michrowska, A. A highly efficient ruthenium catalyst for metathesis reactions. Angew. Chem. Int. Ed. 41, 4038–4040 (2002).
Burns, N. Z., Baran, P. S. & Hoffmann, R. W. Redox economy in organic synthesis. Angew. Chem. Int. Ed. 48, 2854–2867 (2009).
Hoffmann, R. W. Protecting-group-free synthesis. Synthesis 3531–3541 (2006).
Acknowledgements
We thank M. T. Hechavarria Fonseca for early experiments, C. W. Lehmann and J. Rust for X-ray analyses and M. Hannappel for technical assistance. A.M. thanks the Alexander von Humboldt Foundation for a fellowship and Mike Doenhoff for help with the bioassay and for providing the snails (original stock of B. alexandrina supplied by S. Botros). The authors acknowledge generous funding from the Max Planck Society, the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 1234 kb)
Crystallographic data for compound 2
Supplementary information (CIF 16 kb)
Rights and permissions
About this article
Cite this article
Michrowska, A., List, B. Concise synthesis of ricciocarpin A and discovery of a more potent analogue. Nature Chem 1, 225–228 (2009). https://doi.org/10.1038/nchem.215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.215
This article is cited by
-
Applications of Knoevenagel condensation reaction in the total synthesis of natural products
Monatshefte für Chemie - Chemical Monthly (2020)
-
Organocatalytic cascade reactions as a new tool in total synthesis
Nature Chemistry (2010)