Take aim - p955
doi:10.1038/nchem.1521
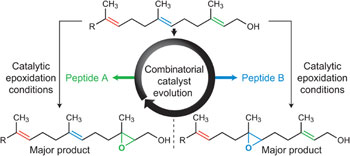
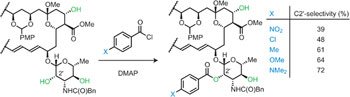
A collection of articles in this issue focuses on the ability to selectively perform a reaction at just one specific site in a complex molecule that contains many other similarly reactive sites.
Full Text - Take aim | PDF (168 KB) - Take aim
Subject terms: Organic chemistry | Synthesis