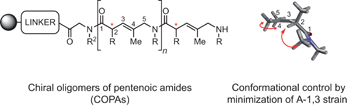
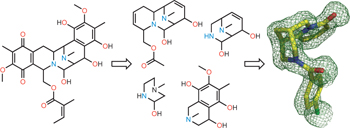
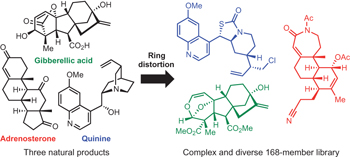
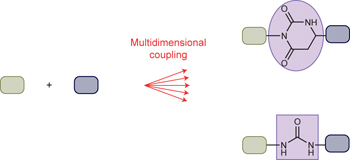
Inspiration comes naturally - p841
doi:10.1038/nchem.2081
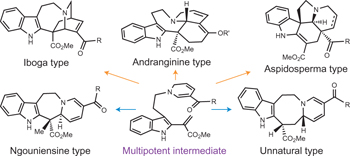
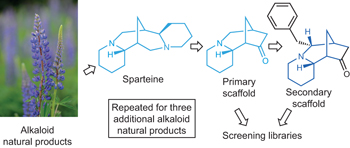
A collection of articles in this issue focuses on attempts to mimic aspects of natural-product biosynthesis for the identification of new drugs.
Full Text - Inspiration comes naturally | PDF (535 KB) - Inspiration comes naturally
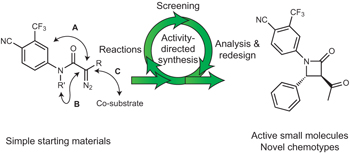
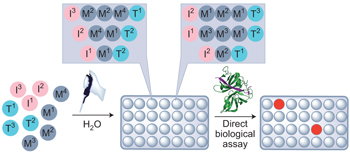
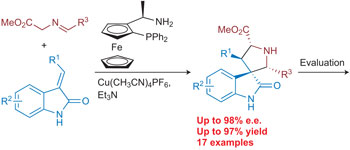
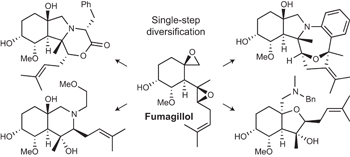
See also: Interview with Jeffrey Bode | Interview with Adam Nelson and Stuart Warriner | News and Views by Lowe | Article by Karageorgis et al. | Article by Huang & Bode