Abstract
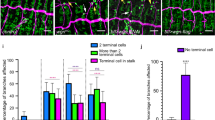
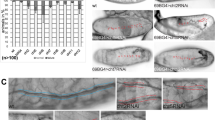
The Drosophila melanogaster tracheal system and the mammalian lung are branching networks of tubular epithelia that convert during late embryogenesis from liquid- to air-filling1,2,3. Little is known about how respiratory-tube size and physiology are coordinated. Here, we show that the Drosophila wurst gene encodes a unique J-domain transmembrane protein highly conserved in metazoa. In wurst mutants, respiratory-tube length is increased and lumen clearance is abolished, preventing gas filling of the airways. Wurst is essential for clathrin-mediated endocytosis4, which is required for size determination and lumen clearance of the airways. wurst recruits heat shock cognate protein 70-4 and clathrin to the apical membrane of epithelial cells. The sequence conservation of the single Wurst orthologues in mice and humans offer new opportunities for genetic studies of clinically relevant lung syndromes caused by the failure of liquid clearance and respiratory-tube size control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Manning, G. & Krasnow, M. in The Development of Drosophila melanogaster. (eds Bate,M. & Martinez Arias, A.) 609–685 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1993).
Olver, R. E., Walters, D. V. & M. Wilson, S. Developmental regulation of lung liquid transport. Annu. Rev. Physiol. 66, 77–101 (2004).
Affolter, M. et al. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11–18 (2003).
Conner, S. D. & Schmid, S. L. Regulated portals of entry into the cell. Nature 422, 37–44 (2003).
Hogan, B. L. M. & Kolodziej, P. A. Organogenesis: molecular mechanisms of tubulogenesis. Nature Rev. Genet. 3, 513–523 (2002).
Davis, G. E. & Senger, D. R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 (2005).
Fischer, H. & Widdicombe, J. H. Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 211, 139–150 (2006).
Metzger, R. J. & Krasnow, M. A. Genetic control of branching morphogenesis. Science 284, 1635–1639 (1999).
Beitel, G. J. & Krasnow, M. A. Genetic control of epithelial tube size in the Drosophila tracheal system. Development 127, 3271–3282 (2000).
O'Brodovich, H. M. Immature epithelial Na+ channel expression is one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome. Proc. Assoc. Am. Physicians 108, 345–355 (1996).
Ungewickell, E. et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature 378, 632–635 (1995).
Bukau, B., Weissman, J. & Horwich, A. Molecular chaperones and protein quality control. Cell 125, 443–451 (2006).
Kelley, W. L. Molecular chaperones: How J domains turn on Hsp70s. Curr. Biol. 9, R305–R308 (1999).
Pellecchia, M. et al. NMR structure of the J-domain and the Gly/Phe-rich region of the Escherichia coli DnaJ chaperone. J. Mol. Biol. 260, 236–250 (1996).
Behr, M., Riedel, D. & Schuh, R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev. Cell 5, 611–620 (2003).
Wu, V. M. et al. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 164, 313–323 (2004).
Tonning, A. et al. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 9, 423–430 (2005).
Devine, W. P. et al. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl Acad. Sci. USA 102, 17014–17019 (2005).
Luschnig, S. et al. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 16, 186–194 (2006).
Wang, S. et al. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 16, 180–185 (2006).
Moussian, B. et al. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133, 163–171 (2006).
Wucherpfennig, T., Wilsch-Brauninger, M. & Gonzalez-Gaitan, M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609–624 (2003).
Bronk, P. et al. Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron 30, 475–488 (2001).
Marks, B. et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231–235 (2001).
Chang, H. C. et al. Hsc70 is required for endocytosis and clathrin function in Drosophila. J. Cell Biol. 159, 477–487 (2002).
Chang, H. C., Hull, M. & Mellman, I. The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J. Cell Biol. 164, 1055–1064 (2004).
Newmyer, S. L., Christensen, A. & Sever, S. Auxilin–dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell 4, 929–940 (2003).
Liu, L., Johnson, W. A. & Welsh, M. J. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc. Natl Acad. Sci. USA 100, 2128–2133 (2003).
Hummler, E. et al. Early death due to defective neonatal lung liquid clearance in a-ENaC-deficient mice. Nature Genetics 12, 325–328 (1996).
Acknowledgements
We thank: C. Samakovlis for communicating unpublished results; M. Affolter, R. Fehon, M. González-Gaitán, L. Liu, B. Moussian, K. Palter, D. F. Ready, U. Schäfer and S. Luschnig for sharing fly stocks and reagents; T. Magin, I. Zinke and B. Fuss for comments on the manuscript; and the members of the Hoch laboratory for helpful discussions. M.B. would like to thank L. Kutschenko, V. Arndt, J. Martini and all members of the Hoch laboratory for technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to M.B. (BE3215) and to M.H. (SFB 645).
Author information
Authors and Affiliations
Contributions
Norther blot, electron microscopic studies and clathrin interaction assay were performed by C. Wingen; molecular analysis of the l(1)G0162 P-element was performed by C. Wolf; all other experiments were performed by M.B.; the wurst project was initiated by M.B. and R.S.; M.H. supervised the research project; M.H. wrote the manuscript; all authors discussed the experimental results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 1950 kb)
Rights and permissions
About this article
Cite this article
Behr, M., Wingen, C., Wolf, C. et al. Wurst is essential for airway clearance and respiratory-tube size control. Nat Cell Biol 9, 847–853 (2007). https://doi.org/10.1038/ncb1611
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1611
This article is cited by
-
Glycosylhydrolase genes control respiratory tubes sizes and airway stability
Scientific Reports (2020)
-
WASH phosphorylation balances endosomal versus cortical actin network integrities during epithelial morphogenesis
Nature Communications (2019)
-
A cross-species approach to identify transcriptional regulators exemplified for Dnajc22 and Hnf4a
Scientific Reports (2017)
-
Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila
Nature Communications (2013)
-
Complex cellular functions of the von Hippel–Lindau tumor suppressor gene: insights from model organisms
Oncogene (2012)