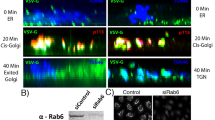
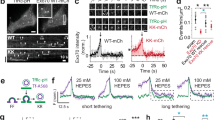
Abstract
The mammalian Golgi apparatus exists as stacks of cisternae that are laterally linked to form a continuous membrane ribbon, but neither the molecular requirements for, nor the purpose of, Golgi ribbon formation are known. Here, we demonstrate that ribbon formation is mediated by specific membrane-fusion events that occur during Golgi assembly, and require the Golgi proteins GM130 and GRASP65. Furthermore, these GM130 and GRASP65-dependent lateral cisternal-fusion reactions are necessary to achieve uniform distribution of enzymes in the Golgi ribbon. The membrane continuity created by ribbon formation facilitates optimal processing conditions in the biosynthetic pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rios, R. M. & Bornens, M. The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 15, 60–66 (2003).
Thyberg, J. & Moskalewski, S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 246, 263–279 (1999).
Cole, N. B., Sciaky, N., Marotta, A., Song, J. & Lippincott-Schwartz, J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631–650 (1996).
Storrie, B. et al. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505–1521 (1998).
Akhmanova, A. et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104, 923–935 (2001).
Diao, A., Rahman, D., Pappin, D. J., Lucocq, J. & Lowe, M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol 160, 201–212 (2003).
Fridmann-Sirkis, Y., Siniossoglou, S. & Pelham, H. R. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol 5, 18 (2004).
Larocca, M. C. et al. AKAP350 interaction with cdc42 interacting protein 4 at the golgi apparatus. Mol Biol Cell 15, 2771–2781 (2004).
Perez, F. et al. CLIPR-59, a new trans-Golgi/TGN cytoplasmic linker protein belonging to the CLIP-170 family. J Cell Biol 156, 631–642 (2002).
Walenta, J. H., Didier, A. J., Liu, X. & Kramer, H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J. Cell Biol. 152, 923–934 (2001).
Rios, R. M., Sanchis, A., Tassin, A. M., Fedriani, C. & Bornens, M. GMAP-210 recruits γ-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118, 323–335 (2004).
Zolov, S. N. & Lupashin, V. V. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J. Cell Biol. 168, 747–759 (2005).
Shorter, J. & Warren, G. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 146, 57–70 (1999).
Cole, N. B. et al. Diffusional mobility of Golgi proteins in membranes of living cells. Science 273, 797–801 (1996).
Puri, S. & Linstedt, A. D. Capacity of the Golgi apparatus for biogenesis from the endoplasmic reticulum. Mol. Biol. Cell 14, 5011–5018 (2003).
de Graffenried, C. L. & Bertozzi, C. R. The roles of enzyme localisation and complex formation in glycan assembly within the Golgi apparatus. Curr. Opin. Cell Biol. 16, 356–363 (2004).
Spiro, R. G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12, 43R–56R (2002).
Nakamura, N., Lowe, M., Levine, T. P., Rabouille, C. & Warren, G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89, 445–455 (1997).
Puthenveedu, M. A. & Linstedt, A. D. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc. Natl Acad. Sci. USA 101, 1253–1256 (2004).
Barr, F. A., Nakamura, N. & Warren, G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 17, 3258–3268 (1998).
Sutterlin, C., Polishchuk, R., Pecot, M. & Malhotra, V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell 16, 3211–3222 (2005).
Wang, Y., Seemann, J., Pypaert, M., Shorter, J. & Warren, G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 22, 3279–3290 (2003).
Sonnichsen, B. et al. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140, 1013–1021 (1998).
Seemann, J., Jokitalo, E., Pypaert, M. & Warren, G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407, 1022–1026 (2000).
Roti, E. C. et al. Interaction with GM130 during HERG ion channel trafficking. Disruption by type-2 congenital long QT syndrome mutations. Human Ether-a-go-go-Related Gene. J. Biol Chem. 277, 47779–47785 (2002).
Preisinger, C. et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14–3–3ζ. J. Cell Biol. 164, 1009–1020 (2004).
Puthenveedu, M. A. & Linstedt, A. D. Evidence that Golgi structure depends on a p115 activity that is independent of the vesicle tether components giantin and GM130. J. Cell Biol. 155, 227–238 (2001).
Vasile, E., Perez, T., Nakamura, N. & Krieger, M. Structural integrity of the Golgi is temperature sensitive in conditional-lethal mutants with no detectable GM130. Traffic 4, 254–272 (2003).
Kondylis, V. & Rabouille, C. A novel role for dp115 in the organization of tER sites in Drosophila. J. Cell Biol. 162, 185–198 (2003).
Shorter, J. et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 18, 4949–4960 (1999).
Linstedt, A. D. Stacking the cisternae. Curr. Biol. 9, R893–R896 (1999).
Kondylis, V., Spoorendonk, K. M. & Rabouille, C. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol. Biol. Cell 16, 4061–4072 (2005)
Yano, H. et al. Distinct functional units of the Golgi complex in Drosophila cells. Proc. Natl Acad. Sci. USA 102, 13467–13472 (2005).
Altmann, F., Fabini, G., Ahorn, H. & Wilson, I. B. Genetic model organisms in the study of N-glycans. Biochimie 83, 703–712 (2001).
Sutterlin, C., Hsu, P., Mallabiabarrena, A. & Malhotra, V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109, 359–369 (2002).
Kano, F. et al. MEK and Cdc2 kinase are sequentially required for Golgi disassembly in MDCK cells by the mitotic Xenopus extracts. J. Cell Biol. 149, 357–368 (2000).
Puthenveedu, M. A. & Linstedt, A. D. In search of an essential step during mitotic Golgi disassembly and inheritance. Exp. Cell Res. 271, 22–27 (2001).
Norman, T. C. et al. Genetic selection of peptide inhibitors of biological pathways. Science 285, 591–595 (1999).
Natarajan, R. & Linstedt, A. D. A cycling cis-Golgi protein mediates endosome-to-Golgi traffic. Mol. Biol. Cell 15, 4798–4806 (2004).
Puri, S., Bachert, C., Fimmel, C. J. & Linstedt, A. D. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 3, 641–653 (2002).
Wright, R. Transmission electron microscopy of yeast. Microsc Res Tech 51, 496–510 (2000).
Jesch, S. A. & Linstedt, A. D. The Golgi and endoplasmic reticulum remain independent during mitosis in HeLa cells. Mol Biol Cell 9, 623–635 (1998).
Acknowledgements
We thank J. Suhan for the electron microscopy, T. Feinstein for the VSV-G–GFP cells and for assistance with GRASP65 knockdown, T. Lee for helpful discussions, J. White for the GalNAc-T2 cells, O. Weisz for the anti-VSV-G antibody, and V. Malhotra and G. Warren for the GRASP65 antibodies. Funding was provided by grants RSG-03-148-01-CSM and GM-56779-02 to A.D.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2 and S3 (PDF 262 kb)
Supplementary Information
Supplementary Movie S1 (MOV 788 kb)
Supplementary Information
Supplementary Movie S2 (MOV 1000 kb)
Rights and permissions
About this article
Cite this article
Puthenveedu, M., Bachert, C., Puri, S. et al. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol 8, 238–248 (2006). https://doi.org/10.1038/ncb1366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1366
This article is cited by
-
The Golgi stacking protein GRASP55 is targeted by the natural compound prodigiosin
Cell Communication and Signaling (2023)
-
The crescent-like Golgi ribbon is shaped by the Ajuba/PRMT5/Aurora-A complex-modified HURP
Cell Communication and Signaling (2023)
-
Structure modeling hints at a granular organization of the Golgi ribbon
BMC Biology (2022)
-
GM130 regulates pulmonary surfactant protein secretion in alveolar type II cells
Science China Life Sciences (2022)
-
Altered cellular localisation and expression, together with unconventional protein trafficking, of prion protein, PrPC, in type 1 diabetes
Diabetologia (2021)