Abstract
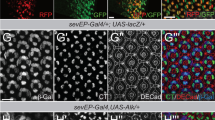
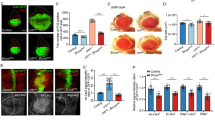
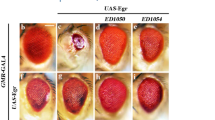
Merlin, the protein product of the Neurofibromatosis type-2 gene, acts as a tumour suppressor in mice and humans. Merlin is an adaptor protein with a FERM domain and it is thought to transduce a growth-regulatory signal. However, the pathway through which Merlin acts as a tumour suppressor is poorly understood. Merlin, and its function as a negative regulator of growth, is conserved in Drosophila, where it functions with Expanded, a related FERM domain protein. Here, we show that Drosophila Merlin and Expanded are components of the Hippo signalling pathway, an emerging tumour-suppressor pathway. We find that Merlin and Expanded, similar to other components of the Hippo pathway, are required for proliferation arrest and apoptosis in developing imaginal discs. Our genetic and biochemical data place Merlin and Expanded upstream of Hippo and identify a pathway through which they act as tumour-suppressor genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Johnston, L. A. & Gallant, P. Control of growth and organ size in Drosophila. Bioessays 24, 54–64 (2002).
Conlon, I. & Raff, M. Size control in animal development. Cell 96, 235–244 (1999).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Ryoo, H. D. & Steller, H. Hippo and its mission for growth control. Nature Cell Biol. 5, 853–855 (2003).
Hay, B. A. & Guo, M. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev. Cell 5, 361–363 (2003).
Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. & Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nature Cell Biol. 5, 914–920 (2003).
Pantalacci, S., Tapon, N. & Leopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nature Cell Biol. 5, 921–927 (2003).
Wu, S., Huang, J., Dong, J. & Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456 (2003).
Harvey, K. F., Pfleger, C. M. & Hariharan, I. K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467 (2003).
Jia, J., Zhang, W., Wang, B., Trinko, R. & Jiang, J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514–2519 (2003).
Tapon, N. et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002).
Kango-Singh, M. et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002).
Justice, R. W., Zilian, O., Woods, D. F., Noll, M. & Bryant, P. J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995).
Xu, T., Wang, W., Zhang, S., Stewart, R. A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995).
Lai, Z. C. et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120, 675–685 (2005).
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 (2005).
Chan, E. H. et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 (2005).
Rouleau, G. A. et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363, 515–521 (1993).
Trofatter, J. A. et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 75, 826 (1993).
McClatchey, A. I. Merlin and ERM proteins: unappreciated roles in cancer development? Nature Rev. Cancer 3, 877–883 (2003).
McClatchey, A. I. et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 12, 1121–1133 (1998).
Lallemand, D., Curto, M., Saotome, I., Giovannini, M. & McClatchey, A. I. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 17, 1090–1100 (2003).
Lutchman, M. & Rouleau, G. A. The neurofibromatosis type 2 gene product, schwannomin, suppresses growth of NIH 3T3 cells. Cancer Res. 55, 2270–2274 (1995).
Xiao, G. H., Chernoff, J. & Testa, J. R. NF2: the wizardry of merlin. Genes Chromosom. Cancer 38, 389–399 (2003).
Bretscher, A., Edwards, K. & Fehon, R. G. ERM proteins and merlin: integrators at the cell cortex. Nature Rev. Mol. Cell. Biol. 3, 586–599 (2002).
Rong, R., Tang, X., Gutmann, D. H. & Ye, K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc. Natl Acad. Sci. USA 101, 18200–18205 (2004).
McCartney, B. M., Kulikauskas, R. M., LaJeunesse, D. R. & Fehon, R. G. The Neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development 127, 1315–1324 (2000).
Boedigheimer, M. & Laughon, A. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development 118, 1291–1301 (1993).
LaJeunesse, D. R., McCartney, B. M. & Fehon, R. G. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J. Cell Biol. 141, 1589–1599 (1998).
Boedigheimer, M. J., Nguyen, K. P. & Bryant, P. J. Expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev. Genet. 20, 103–110 (1997).
Blaumueller, C. M. & Mlodzik, M. The Drosophila tumor suppressor expanded regulates growth, apoptosis, and patterning during development. Mech. Dev. 92, 251–262 (2000).
Baker, N. E. Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12, 499–507 (2001).
Wolff, T. & Ready, D. F. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development 113, 841–850 (1991).
Potter, C. J., Huang, H. & Xu, T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105, 357–368 (2001).
Tapon, N., Ito, N., Dickson, B. J., Treisman, J. E. & Hariharan, I. K. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105, 345–355 (2001).
Richardson, H., O'Keefe, L. V., Marty, T. & Saint, R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development 121, 3371–3379 (1995).
Jones, L., Richardson, H. & Saint, R. Tissue-specific regulation of cyclin E transcription during Drosophila melanogaster embryogenesis. Development 127, 4619–4630 (2000).
Hay, B. A., Wassarman, D. A. & Rubin, G. M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83, 1253–1262 (1995).
Ryoo, H. D., Bergmann, A., Gonen, H., Ciechanover, A. & Steller, H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nature Cell Biol. 4, 432–438 (2002).
Niehrs, C. & Meinhardt, H. Modular feedback. Nature 417, 35–36 (2002).
Turenchalk, G. S., St John, M. A., Tao, W. & Xu, T. The role of lats in cell cycle regulation and tumorigenesis. Biochim. Biophys. Acta 1424, M9–M16 (1999).
St John, M. A. et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nature Genet. 21, 182–186 (1999).
Tao, W. et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nature Genet. 21, 177–181 (1999).
Dan, I., Watanabe, N. M. & Kusumi, A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230 (2001).
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993).
Newsome, T. P., Asling, B. & Dickson, B. J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 (2000).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Struhl, G. & Basler, K. Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 (1993).
Acknowledgements
We thank R.G. Fehon, A. Laughon, M. Mlodzik, B. Hay, D. Pan, P. Bryant, the Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank (University of Iowa) for fly stocks, antibodies and plasmids. We thank K.K. Norga for his help with the isolation of new ex alleles. We thank K.Dunner for technical help with the scanning electron microscopy analysis, which were performed, along with DNA sequencing, at the M. D. Anderson core facilities supported by a National Cancer Institute Cancer Center support grant. We also thank L. McCord for her help with artwork. Special thanks to M. Acar for his invaluable advice and help with the cell-culture experiments. We thank the members of the X. Chen laboratory for their help with antibody production. We thank R. Behringer, H.J. Bellen, A. Bergmann, K.-W. Choi, V. Dion, N. Giagtzoglou, P.R. Hiesinger, G. Lozano, G. Mardon, R. Johnson, J. Kunz and members of the Halder Lab for discussions. This work was supported by a National Institutes of Health grant to G.H, The Odyssey Fellowship and The Theodore N. Law Award for Scientific Achievement to M.K.S., and a BRASS Scholarship to E.H. H.J.N. is supported by HHMI and by a NIH Medical Genetics Research Fellowship Program grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2 and S3 (PDF 377 kb)
Rights and permissions
About this article
Cite this article
Hamaratoglu, F., Willecke, M., Kango-Singh, M. et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8, 27–36 (2006). https://doi.org/10.1038/ncb1339
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1339
This article is cited by
-
Lipid kinase PIP5Kα contributes to Hippo pathway activation via interaction with Merlin and by mediating plasma membrane targeting of LATS1
Cell Communication and Signaling (2023)
-
Cellular mechanisms of heterogeneity in NF2-mutant schwannoma
Nature Communications (2023)
-
Cell adhesion molecule KIRREL1 is a feedback regulator of Hippo signaling recruiting SAV1 to cell-cell contact sites
Nature Communications (2022)
-
The Hippo pathway kinases LATS1 and LATS2 attenuate cellular responses to heavy metals through phosphorylating MTF1
Nature Cell Biology (2022)
-
YAP induces an oncogenic transcriptional program through TET1-mediated epigenetic remodeling in liver growth and tumorigenesis
Nature Genetics (2022)