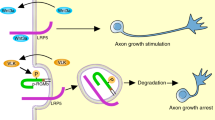
Abstract
Neurite extension is essential for wiring the nervous system during development. Although several factors are known to regulate neurite outgrowth, the underlying mechanisms remain unclear. Here, we provide evidence for a role of phosphatidylinositol transfer protein-α (PITPα) in neurite extension in response to netrin-1, an extracellular guidance cue. PITPα interacts with the netrin receptor DCC (deleted in colorectal cancer) and neogenin. Netrin-1 stimulates PITPα binding to DCC and to phosphatidylinositol (5) phosphate [PI(5)P], increases its lipid-transfer activity and elevates hydrolysis of phosphatidylinositol bisphosphate (PIP2). In addition, the stimulated PIP2 hydrolysis requires PITPα. Furthermore, cortical explants of PITPα mutant mice are defective in extending neurites in response to netrin-1. Commissural neurons from chicken embryos expressing a dominant-negative PITPα mutant show reduced axon outgrowth. Morpholino-mediated knockdown of PITPα expression in zebrafish embryos leads to dose-dependent defects in motor-neuron axons and reduced numbers of spinal-cord neurons. Taken together, these results identify a crucial role for PITPα in netrin-1-induced neurite outgrowth, revealing a signalling mechanism for DCC/neogenin and PITPα regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Chan, S. S. et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87, 187–195 (1996).
Kolodziej, P. A. et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996).
Hiramoto, M., Hiromi, Y., Giniger, E. & Hotta, Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature 406, 886–889 (2000).
Ren, X. R. et al. Focal adhesion kinase in netrin-1 signaling. Nature Neurosci. 7, 1204–1212 (2004).
Ming, G. et al. Phospholipase C-γ and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 23, 139–148 (1999).
Forcet, C. et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 417, 443–447 (2002).
Li, X., Saint-Cyr-Proulx, E., Aktories, K. & Lamarche-Vane, N. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (Deleted in Colorectal Cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 277, 15207–15214 (2002).
Hu, G. et al. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 11, 2701–2714 (1997).
Culotti, J. G. & Merz, D. C. DCC and netrins. Curr. Opin. Cell Biol. 10, 609–613 (1998).
Kennedy, T. E. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem. Cell Biol. 78, 569–575 (2000).
Stein, E. & Tessier-Lavigne, M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938 (2001).
Stein, E., Zou, Y., Poo, M. & Tessier-Lavigne, M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science 291, 1976–1982 (2001).
Allen-Baume, V., Segui, B. & Cockcroft, S. Current thoughts on the phosphatidylinositol transfer protein family. FEBS Lett. 531, 74–80 (2002).
Hara, S., Swigart, P., Jones, D. & Cockcroft, S. The first 5 amino acids of the carboxyl terminus of phosphatidylinositol transfer protein (PITP) alpha play a critical role in inositol lipid signaling. Transfer activity of PITP is essential but not sufficient for restoration of phospholipase C signaling. J. Biol. Chem. 272, 14908–14913 (1997).
Graef, I. A. et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003).
Brose, K. & Tessier-Lavigne, M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr. Opin. Neurobiol. 10, 95–102 (2000).
Finger, J. H. et al. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J. Neurosci. 22, 10346–10356 (2002).
Schouten, A. et al. Structure of apo-phosphatidylinositol transfer protein alpha provides insight into membrane association. EMBO J. 21, 2117–2121 (2002).
Yoder, M. D. et al. Structure of a multifunctional protein. Mammalian phosphatidylinositol transfer protein complexed with phosphatidylcholine. J. Biol. Chem. 276, 9246–9252 (2001).
Wang, Q. et al. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol. 160, 565–575 (2003).
Van Paridon, P. A., Gadella, T. W. Jr & Wirtz, K. W. The effect of polyphosphoinositides and phosphatidic acid on the phosphatidylinositol transfer protein from bovine brain: a kinetic study. Biochim. Biophys. Acta 943, 76–86 (1988).
Hamilton, B. A. et al. The vibrator mutation causes neurodegeneration via reduced expression of PITPα: positional complementation cloning and extragenic suppression. Neuron 18, 711–722 (1997).
Metin, C., Deleglise, D., Serafini, T., Kennedy, T. E. & Tessier-Lavigne, M. A role for netrin-1 in the guidance of cortical efferents. Development 124, 5063–5074 (1997).
Braisted, J. E. et al. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J. Neurosci. 20, 5792–5801 (2000).
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Nasevicius, A. & Ekker, S. C. Effective targeted gene 'knockdown' in zebrafish. Nature Genet. 26, 216–220 (2000).
Mawdsley, D. J. et al. The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish. Dev. Biol. 269, 302–315 (2004).
Morgan, C. P. et al. Phosphorylation of a distinct structural form of PITP at Ser166 by protein kinase C disrupts receptor-mediated phospholipase C signalling by inhibiting delivery of phosphatidylinositol to membranes. J. Biol. Chem. 279, 47159–47171 (2004).
Takei, K., Shin, R. M., Inoue, T., Kato, K. & Mikoshiba, K. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science 282, 1705–1708 (1998).
Li, H. S. et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96, 807–818 (1999).
Ren, X. R. et al. Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J. Cell Biol. 152, 971–984 (2001).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Richards, L. J., Koester, S. E., Tuttle, R. & O'Leary, D. D. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J. Neurosci. 17, 2445–2458 (1997).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Acknowledgements
We are grateful to Drs B.A. Hamilton (University of California at San Diego), B. Vogelstein (Johns Hopkins Medical School), M. Tessier-Lavigne, (Stanford University), J.Y. Wu and Y. Rao (Washington University) for reagents. We thank Dr R.-B. Markowitz (Medical College of Georgia) for reading the manuscript. This study was supported by grants from the National Institutes of Health (NS35900 for S.L.A.; DC006140 for D.K.; NS40480, NS045710 and NS44521 for L.M.; GM63861 and AR48120 for W.-C.X.; and the Philip Morris Research Program and Muscular Dystrophy Association for L.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2 and S3 (PDF 382 kb)
Rights and permissions
About this article
Cite this article
Xie, Y., Ding, YQ., Hong, Y. et al. Phosphatidylinositol transfer protein-α in netrin-1-induced PLC signalling and neurite outgrowth. Nat Cell Biol 7, 1124–1132 (2005). https://doi.org/10.1038/ncb1321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1321
This article is cited by
-
Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis
Nature Communications (2019)
-
Large-scale microfluidic gradient arrays reveal axon guidance behaviors in hippocampal neurons
Microsystems & Nanoengineering (2017)
-
Phosphatidylinositol transfer protein-α in platelets is inconsequential for thrombosis yet is utilized for tumor metastasis
Nature Communications (2017)
-
Optogenetic activation of axon guidance receptors controls direction of neurite outgrowth
Scientific Reports (2016)
-
VPS35-deficiency results in an impaired AMPA receptor trafficking and decreased dendritic spine maturation
Molecular Brain (2015)