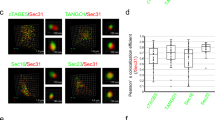
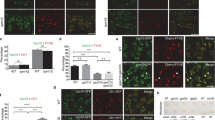
Abstract
Soluble secretory proteins are first translocated across endoplasmic reticulum (ER) membranes and folded in a specialized ER luminal environment. Fully folded and assembled secretory cargo are then segregated from ER-resident proteins into COPII-derived vesicles or tubular elements for anterograde transport. Mechanisms of bulk-flow, ER-retention and receptor-mediated export have been suggested to operate during this transport step, although these mechanisms are poorly understood1,2,3,4,5,6,7. In yeast, there is evidence to suggest that Erv29p functions as a transmembrane receptor for the export of certain soluble cargo proteins including glycopro-α-factor (gpαf), the precursor of α-factor mating pheromone8. Here we identify a hydrophobic signal within the pro-region of gpαf that is necessary for efficient packaging into COPII vesicles and for binding to Erv29p. When fused to Kar2p, an ER-resident protein, the pro-region sorting signal was sufficient to direct Erv29p-dependent export of the fusion protein into COPII vesicles. These findings indicate that specific motifs within soluble secretory proteins function in receptor-mediated export from the ER. Moreover, positive sorting signals seem to predominate over potential ER-retention mechanisms that may operate in localizing ER-resident proteins such as Kar2p.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Wieland, F. T., Gleason, M. L., Serafini, T. A. & Rothman, J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50, 289–300 (1987).
Kuehn, M. J., Herrmann, J. M. & Schekman, R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391, 187–190 (1998).
Appenzeller, C., Andersson, H., Kappeler, F. & Hauri, H. P. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nature Cell Biol. 1, 330–334 (1999).
Martinez-Menarguez, J. A., Geuze, H. J., Slot, J. W. & Klumperman, J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell 98, 81–90 (1999).
Muniz, M., Nuoffer, C., Hauri, H. -P. & Riezman, H. The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J. Cell Biol. 148, 925–930 (2000).
Polishchuk, E. V., Di Pentima, A., Luini, A. & Polishchuk, R. S. Mechanism of constitutive export from the Golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-Golgi network tubular domains. Mol. Biol. Cell 14, 4470–4485 (2003).
Barlowe, C. Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol. 13, 295–300 (2003).
Belden, W. J. & Barlowe, C. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science 294, 1528–1531 (2001).
Kurjan, J. & Herskowitz, I. Structure of a yeast pheromone gene (MFα): a putative α-factor precursor contains four tandem copies of mature α-factor. Cell 30, 933–943 (1982).
Julius, D., Blair, L., Brake, A., Sprague, G. & Thorner, J. Yeast α-factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32, 839–852 (1983).
Otte, S. et al. Erv41p and Erv46p: New components of COPII vesicles involved in transport between the ER and Golgi complex. J. Cell Biol. 152, 503–517 (2001).
Caplan, S., Green, E., Rocco, J. & Kurjan, J. Glycosylation and structure of the yeast MFα1 α-factor precursor is important for efficient transport through the secretory pathway. J. Bacteriol. 173, 627–635 (1991).
Baker, D., Hicke, L., Rexach, M., Schleyer, M. & Schekman, R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 54, 335–344 (1988).
Barlowe, C. et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell 77, 895–907 (1994).
McCracken, A. A. & Brodsky, J. L. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J. Cell Biol. 132, 291–298 (1996).
Caldwell, S. R., Hill, K. J. & Cooper, A. A. Degradation of endoplasmic reticulum substrates requires transport between the ER and Golgi. J. Biol. Chem. 276, 23296–23302 (2001).
Kaiser, C. A., Gimeno, R. E. & Shaywitz, D. A. in Yeast III (eds Pringle, J. R., Broach, J. R. & Jones, E. W.) 91–227 (Cold Spring Harbor Laboratory Press, 1997).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response Cell 90, 1031–1039 (1997).
Belden, W. J. & Barlowe, C. Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell 12, 957–969 (2001).
Oka, C. et al. Human lysozyme secretion increased by α-factor pro-sequence in Pichia pastoris. Biosci. Biotechnol. Biochem. 63, 1977–1983 (1999).
Yeung, T., Barlowe, C. & Schekman, R. Uncoupled packaging of targeting and cargo molecules during transport vesicle budding from the endoplasmic reticulum. J. Biol. Chem. 270, 30567–30570 (1995).
Ellgaard, L. & Helenius, A. Quality control in the endoplasmic reticulum. Nature Rev. Mol. Cell Biol. 4, 181–191 (2003).
Dean, N. & Pelham, H. R. B. Recycling of proteins from the Golgi compartment to the ER in yeast. J. Cell Biol. 111, 369–377 (1990).
Semenza, J. C., Hardwick, K. G., Dean, N. & Pelham, H. R. B. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61, 1349–1357 (1990).
Elrod-Erickson, M. J. & Kaiser, C. A. Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol. Biol. Cell 7, 1043–1058 (1996).
Eder, J. & Fersht, A. R. Pro-sequence-assisted protein folding. Mol. Microbiol. 16, 609–614 (1995).
Garrett, P. J. et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor α. Cell 110, 763–773 (2002).
Belden, W. J. & Barlowe, C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J. Biol. Chem. 271, 26939–26946 (1996).
Tachibana, C. & Stevens, T. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol. Cell. Biol. 12, 4601–4611 (1992).
Powers, J. & Barlowe, C. Transport of Axl2p depends on Erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J. Cell Biol. 142, 1209–1222 (1998).
Acknowledgements
We thank E. Harris and Y. Jun for their assistance in the early stages of this study and T. Stevens for providing anti-Pdi1p serum. We also thank J. Flanagan and M. Heidtman for their comments on this manuscript. This work was supported by a grant from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information,
Fig. S1, Fig. S2 and Table 1 (PDF 112 kb)
Rights and permissions
About this article
Cite this article
Otte, S., Barlowe, C. Sorting signals can direct receptor-mediated export of soluble proteins into COPII vesicles. Nat Cell Biol 6, 1189–1194 (2004). https://doi.org/10.1038/ncb1195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1195
This article is cited by
-
Constitutive and extracellular expression of pectin methylesterase from Pectobacterium chrysanthemi in Pichia pastoris
3 Biotech (2022)
-
Disulfide bond engineering of AppA phytase for increased thermostability requires co-expression of protein disulfide isomerase in Pichia pastoris
Biotechnology for Biofuels (2021)
-
Design of an improved universal signal peptide based on the α-factor mating secretion signal for enzyme production in yeast
Cellular and Molecular Life Sciences (2021)
-
An improved secretion signal enhances the secretion of model proteins from Pichia pastoris
Microbial Cell Factories (2018)
-
The role of protein–protein interactions in the intracellular traffic of the potassium channels TASK-1 and TASK-3
Pflügers Archiv - European Journal of Physiology (2015)