Abstract
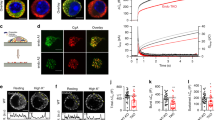
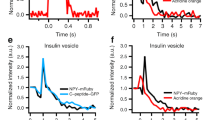
Electrophysiological studies in some secretory and non-secretory cells have identified an extensive form of calcium-induced exocytosis that is rapid (hundreds of milliseconds), insensitive to tetanus toxin and distinct from regulated secretion. We have now identified a marker of the process, desmoyokin-AHNAK, in a clonal derivative of the neuronal cell line, PC12. In resting cells, desmoyokin-AHNAK is localized within the lumen of specific vesicles, but appears on the cell surface during stimulation. Desmoyokin-AHNAK-positive vesicles exist in a variety of cells and tissues and are distinct from the endoplasmic reticulum, Golgi, trans-Golgi, endosomes and lysosomes, and from Glut4 and constitutive secretion vesicles. They seem to be involved in two models of plasmalemma enlargement: differentiation and membrane repair. We therefore propose that these vesicles should be called 'enlargosomes'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jahn, R. & Südhof, T. C. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68, 863–911 (1999).
Kasai, H. Comparative biology of Ca2+-dependent exocytosis: implications of kinetic diversity for secretion function. Trends Neurosci. 22, 88–93 (1999).
Coorssen, J. R., Schmitt, H. & Almers, W. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 15, 3787–3791 (1996).
Ninomiya, Y., Kishimoto, T., Miyashita, Y. & Kasai, H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J. Biol. Chem. 271, 17751–17754 (1996).
Kasai, H. et al. Multiple and diverse exocytosis in wild-type and defective PC12 cells. Proc. Natl Acad. Sci. USA 96, 945–949 (1999).
Andrews, N. W. Regulated secretion of conventional lysosomes. Trends Cell. Biol. 10, 316–321 (2000).
Clementi, E., Racchetti, G., Zacchetti, D., Panzeri, M. C. & Meldolesi, J. Differential expression of markers and activities in a group of PC12 nerve cell clones. Eur. J. Neurosci. 4, 944–953 (1992).
Sussman, J., Stokoe, D., Ossina, N. & Shtivelmann, E. Protein kinase B phosphorylates AHNAK and regulates its subcellular localization. J. Cell Biol. 154, 1019–1030 (2001).
Sköldberg, F. et al. Identification of AHNAK as a novel autoantigen in systemic lupus erythematosus. Biochem. Biophys. Res. Commun. 291, 951–958 (2002).
Shtivelman, E. & Bishop, J. M. The human gene AHNAK encodes a large phosphoprotein located primarily in the nucleus. J. Cell Biol. 120, 625–630 (1993).
Piper, R. C. & Luzio, J. P. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2, 612–621 (2001).
Thoidis, G. & Kandrov, K. V. A Glut4-vesicle marker protein, insulin-responsive aminopeptidase, is localized in a novel vesicular compartment in PC12 cells. Traffic 2, 577–587 (2001).
Kaether, C., Salm, T., Glombik, M., Almers, W. & Gerdes, H. H. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur. J. Cell Biol. 74, 133–142 (1997).
Malosio, M. L. et al. Neurosecretory cells without neurosecretion: evidence of an independently regulated trait of the cell phenotype. J. Physiol. (Lond.) 520, 43–52 (1999).
Paolucci, P., Rovere, P., De Nadai, C., Manfredi, A. A. & Clementi, E. Nitric oxide inhibits the tumor necrosis factor alpha-regulated endocytosis of human dendritic cells in a cyclic GMP-dependent way. J. Biol. Chem. 275, 19638–19644 (2000).
McNeil, P. L. & Terasaki, M. Coping with the inevitable: how cells repair a torn surface membrane. Nature Cell Biol. 3, E124–E129 (2001).
Reddy, A., Caler, E. V. & Andrews, N. W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Hashimoto, T. et al. Desmoyokin, a 280 kDa keratinocyte plasma membrane-associated protein, is homologous to the protein encoded by human gene AHNAK. J. Cell Sci. 105, 275–286 (1993).
Nie, Z., Ning, W., Amagai, M. & Hashimoto, T. C-terminus of desmoyokin-AHNAK protein is responsible for its translocation between the nucleus and cytoplasm. J. Invest. Dermatol. 114, 1044–1049 (2000).
Hashimoto, T., Gamou, S., Shimizu, N., Kitajima, Y. & Nishikawa, T. Regulation of translocation of the desmoyokin-AHNAK protein to the plasma membrane in keratinocytes by protein kinase C. Exp. Cell Res. 217, 258–266 (1995).
Haase, H. et al. Signalling from β-adrenoreceptor to L-type calcium channel: identification of a novel cardiac protein kinase A target possessing similarities to AHNAK. FASEB J. 13, 2161–2172 (1999).
Sekiya, F., Bae, Y. S., Jhon, D. Y., Hwang, S. C. & Rhee, S. G. AHNAK, a protein that binds and activates phospholipase C-γ1 in the presence of arachidonic acid. J. Biol. Chem. 274, 13900–13907 (1999).
Gentil, B. J. et al. The giant protein AHNAK is a specific target for the calcium- and zinc-binding S100B protein. J. Biol. Chem. 276, 23253–23261 (2001).
Lu, W. et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 (2001).
Ward, D. T., Hammond, T. G. & Harris, H. W. Modulation of vasopressin-elicited water transport by trafficking of aquaporin2-containing vesicles. Annu. Rev. Physiol. 61, 683–697 (1999).
Whitehead, J. P. et al. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J. Biol. Chem. 276, 27816–27824 (2001).
Simpson, F., Whitehead, J. P. & James, D. E. GLUT4—at the cross roads between membrane trafficking and signal transduction. Traffic 2, 2–11 (2001).
Banerjee, A., Shih, T., Alexander, E. A. & Schwartz, J. H. SNARE proteins regulate H+-ATPase redistribution to the apical membrane in rat renal inner medullary collecting duct cells. J. Biol. Chem. 274, 26518–26522 (1999).
Blott, E. J. & Griffiths, G. M. Secretory lysosomes. Nature Rev. Mol. Cell Biol. 3, 122–131 (2002).
Almers, W. & Neher, E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J. Physiol. (Lond.) 386, 205–217 (1987).
Thomas, P. et al. Two types of exocytosis observed in single, rat pancreatic acinar cells. J. Physiol. (Lond.) (in the press).
Xu, T., Binz, T., Niemann, H. & Neher, E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nature Neurosci. 1, 192–200 (1998).
Coco, S. et al. Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)-VAMP7 in neuronal cells: evidence for a novel membrane compartment. J. Neurosci. 19, 9803–9812 (1999).
Hackam, D. J. et al. v-SNARE-dependent secretion is required for phagocytosis. Proc. Natl Acad. Sci. USA 95, 11691–11696 (1998).
Mellman, I. Quo vadis: polarized membrane recycling in motility and phagocytosis. J. Cell Biol. 49, 529–530 (2000).
Steinhardt, R. A., Bi, G. & Alderton, J. M. Cell membrane resealing by vesicular mechanism similar to neurotransmitter release. Science 263, 390–393 (1994).
Togo, T., Alderton, J. M., Bi, G. O. & Steinhardt, R. A. The mechanism of facilitated cell membrane resealing. J. Cell Sci. 112, 719–731 (1999).
Detrait, E. R. et al. Plasmalemmal repair of severed neurites of PC12 cells requires Ca2+ and synaptotagmin. J. Neurosci. Res. 62, 566–573 (2000).
Leoni, C. et al. Neurite extension occurs in the absence of regulated exocytosis in PC12 subclones. Mol. Biol. Cell 10, 2919–2931 (1999).
Schmidt, A., Hannah, M. J. & Huttner, W. B. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J. Cell Biol. 137, 445–458 (1997).
Galfré, G., Howe, S. C., Milstein, C., Butcher, G. W. & Howard, J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature 266, 550–552 (1977).
Rupnik, M. et al. Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proc. Natl Acad. Sci. USA 97, 5627–5632 (2000).
Gatti, G., Podini, P. & Meldolesi, J. Overexpression of calsequestrin in L6 myoblasts: formation of endoplasmic reticulum subdomains and their evolution into discrete vacuoles where aggregates of the protein are specifically accumulated. Mol. Biol. Cell 8, 1789–1803 (1997).
Wilm, M. et al. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379, 466–469 (1996).
Van den Hoff, M. J. B., Moorman, A. F. M. & Lamers, W. H. Electroporation in 'intracellular' buffer increases cell survival. Nucleic Acids Res. 20, 2902 (1992).
Acknowledgements
We thank H. Kasai and his laboratory staff for their pioneering collaboration and advice; E. Shtivelman and M. Rupnik for generous support; M. Matteoli and G.P. Schiavo for advice, A. Lorusso for participating in some experiments; C. Paolucci for FACS analysis, R. Barsacchi for performing the [Ca2+]i measurements; and D. Dunlap for the critical revision of the text. Imaging experiments were performed within Alembic (Advanced Light and Electron Microscopy Bio-Imaging Center), San Raffaele Scientific Institute. This work, supported by grants from Telethon, the European Union (QLG1–02233), CNR (Ag. 2000 and the CNS degenerative diseases), the Cofin Italian University System (2001.053389-033) and the Armenise–Harvard Foundation, was performed in the framework of the Italian MIUR Center of Excellence in Physiopathology of Cell Differentiation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures
SI Figure 1 Distinct labeling patterns of PC12 cells with the anti-dA FEN and KIS antibodies. blotting (D and E). (PDF 1678 kb)
SI Figure 2 Surface appearance of dA in stimulated living cells.
SI Figure 3 Cell types investigated for dA expression
Movie I
The movie illustrates in 3D rendering the distribution of the punctate dA surface staining in a PC12-27 cell first stimulated by caged Ca2+ photolysis and then immunolabeled while still living. (GIF 315 kb)
Movie 2
The movie illustrates in 3D rendering the distribution of neurosecretory and "enlargesomal" vesicles in a wild- type PC12 cell differentiated with NGF. (AVI 2820 kb)
Rights and permissions
About this article
Cite this article
Borgonovo, B., Cocucci, E., Racchetti, G. et al. Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 4, 955–963 (2002). https://doi.org/10.1038/ncb888
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb888
This article is cited by
-
The C3d-fused foot-and-mouth disease vaccine platform overcomes maternally-derived antibody interference by inducing a potent adaptive immunity
npj Vaccines (2022)
-
Plasma membrane integrity in health and disease: significance and therapeutic potential
Cell Discovery (2021)
-
Comparison of Gene Transfection and Cytotoxicity Mechanisms of Linear Poly(amidoamine) and Branched Poly(ethyleneimine) Polyplexes
Pharmaceutical Research (2018)
-
A next generation sequencing based approach to identify extracellular vesicle mediated mRNA transfers between cells
BMC Genomics (2017)
-
Dysferlin regulates cell membrane repair by facilitating injury-triggered acid sphingomyelinase secretion
Cell Death & Disease (2014)