Abstract
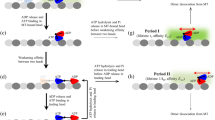
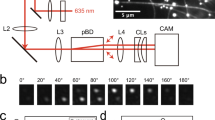
The molecular motor kinesin travels processively along a microtubule in a stepwise manner. Here we have studied the chemomechanical coupling of the hydrolysis of ATP to the mechanical work of kinesin by analysing the individual stepwise movements according to the directionality of the movements. Kinesin molecules move primarily in the forward direction and only occasionally in the backward direction. The hydrolysis of a single ATP molecule is coupled to either the forward or the backward movement. This bidirectional movement is well described by a model of Brownian motion assuming an asymmetric potential of activation energy. Thus, the stepwise movement along the microtubule is most probably due to Brownian motion that is biased towards the forward direction by chemical energy stored in ATP molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vale, R. D., Reese, T. S. & Sheetz, M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50 (1985).
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Block, S. M., Goldstein, L. S. B. & Schnapp, B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348–352 (1990).
Hackney, D. D. Highly processive microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains. Nature 377, 448–450 (1995).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Coppin, C. M., Finer, J. T., Spudich, J. A. & Vale, R. D. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl Acad. Sci. USA 93, 1913–1917 (1996).
Higuchi, H., Muto, E., Inoue, Y. & Yanagida, T. Kinetics of force generation by single kinesin molecules activated by laser photolysis of caged ATP. Proc. Natl Acad. Sci. USA 94, 4395–4400 (1997).
Hua, W., Young, E. C., Fleming, M. L. & Gelles, J. Coupling of kinesin steps to ATP hydrolysis. Nature 388, 390–393 (1997).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Meyhöfer, E. & Howard, J. The force generated by a single kinesin molecule against an elastic load. Proc. Natl Acad. Sci. USA 92, 574–578 (1995).
Coppin, C. M., Pierce, D. W., Hsu, L. & Vale, R. D. The load dependence of kinesin's mechanical cycle. Proc. Natl Acad. Sci. USA 94, 8539–8544 (1997).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997).
Kawaguchi, K. & Ishiwata, S. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 272, 895–899 (2000).
Visscher, K., Schnitzer, M. J. & Block, S. M. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999).
Schnitzer, M. J., Visscher, K. & Block, S. M. Force production by single kinesin motors. Nature Cell Biol. 2, 718–723 (2000).
Nishiyama, M., Muto, E., Inoue, Y., Yanagida, T. & Higuchi, H. Substeps within the 8-nm step of the ATPase cycle of single kinesin molecules. Nature Cell Biol. 3, 425–428 (2001).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer Associates, Sunderland, MA, 2001).
Fisher, M. E. & Kolomeisky, A. B. Simple mechanochemistry describes the dynamics of kinesin molecules. Proc. Natl Acad. Sci. USA 98, 7748–7753 (2001).
Veigel, C. et al. The motor protein myosin-I produces its working stroke in two steps. Nature 398, 530–533 (1999).
Wang, M. D. et al. Force and velocity measured for single molecules of RNA polymerase. Science 282, 902–907 (1998).
Mehta, A. D. et al. Myosin-V is a processive actin-based motor. Nature 400, 590–593 (1999).
Gilbert, S. P., Webb, M. R., Brune, M. & Johnson, K. A. Pathway of processive ATP hydrolysis by kinesin. Nature 373, 671–676 (1995).
Ma, Y. Z. & Taylor, E. W. Mechanism of microtubule kinesin ATPase. Biochemistry 34, 13242–13251 (1995).
Schnitzer, M. J. & Block, S. M. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997).
Rief, M. et al. Myosin-V stepping kinetics: a molecular model for processivity. Proc. Natl Acad. Sci. USA 97, 9482–9486 (2000).
Woledge, R. C., Curtin, N. A. & Homsher, E. Energetic Aspects of Muscle Contraction 167–275 (Academic, London, 1985).
Tomishige, M. & Vale, R. D. Controlling kinesin by reversible disulfide cross-linking: identifying the motility-producing conformational change. J. Cell Biol. 151, 1081–1092 (2000).
Hirose, K., Lockhart, A., Cross, R. A. & Amos, L. A. Three-dimensional cryoelectron microscopy of dimeric kinesin and ncd motor domains on microtubules. Proc. Natl Acad. Sci. USA 93, 9539–9544 (1996).
Kawaguchi, K. & Ishiwata, S. Nucleotide-dependent single- to double-headed binding of kinesin. Science 291, 667–669 (2001).
Rice, S. et al. A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784 (1999).
Kikkawa, M. et al. Switch-based mechanism of kinesin motors. Nature 411, 439–445 (2001).
Romberg, L. & Vale, R. D. Chemomechanical cycle of kinesin differs from that of myosin. Nature 361, 168–170 (1993).
Sosa, H., Peterman, E. J. G., Moerner, W. E. & Goldstein, L. S. B. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nature. Struct. Biol. 8, 540–544 (2001).
Kitamura, K., Tokunaga, M., Iwane, A. H. & Yanagida, T. A single myosin head moves along an actin filament with regular steps of 5.3 nanometres. Nature 397, 129–134 (1999).
Vale, R. D. & Milligan, R. A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Inoue, Y., Iwane, A. H., Miyai, T., Muto, E. & Yanagida, T. Motility of single one-headed kinesin molecules along microtubules. Biophys. J. 81, 2838–2850 (2001).
Okada, Y. & Hirokawa, N. Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc. Natl Acad. Sci. USA 97, 640–645 (2000).
Rogers, K. R. et al. KIF1D is a fast non-processive kinesin that demonstrates novel K-loop-dependent mechanochemistry. EMBO J. 20, 5101–5113 (2001).
Tucker, C. & Goldstein, L. S. B. Probing the kinesin-microtubule interaction. J. Biol. Chem. 272, 9481–9488 (1997).
Nogales, E., Wolf, S. G. & Downing, K. H. Structure of the αβ tubulin dimer by electron crystallography. Nature 391, 199–203 (1998).
Feynman, R. P. in The Feynman Lectures on Physics Vol. I (eds Feynman, R. P., Leighton, R. B. & Sands, M. L.) (Addison-Wesley, Reading, MA, 1963).
Oosawa, F. Sliding of actin filament on myosin and a flexible ratchet. Jikeikai. Med. J. 36, 219–231 (1989).
Vale, R. D. & Oosawa, F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv. Biophys. 26, 97–134 (1990).
Hirakawa, E., Higuchi, H. & Toyoshima, Y. Y. Processive movement of single 22S dynein molecules occurs only at low ATP concentrations. Proc. Natl Acad. Sci. USA 97, 2533–2537 (2000).
Kojima, H., Kikumoto, M., Sakakibara, H. & Oiwa, K. Mechanical properties of single-headed processive motor, inner-arm dynein subspecies-c of Chlamydomonas studied at the single molecule level. J. Biol. Phys. (in the press).
Acknowledgements
We thank Y. Ishii, F. Oosawa, Y. Inoue and colleagues of Single Molecule Processes Project, and Osaka University for discussions; J. West, E. Muto, H. Kojima and Y. Taniguchi for critically reading the manuscript. This work was partially supported by JSPS Research Fellowships for Young Scientists (M.N.).Correspondence and requests for materials should be addressed to H.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nishiyama, M., Higuchi, H. & Yanagida, T. Chemomechanical coupling of the forward and backward steps of single kinesin molecules. Nat Cell Biol 4, 790–797 (2002). https://doi.org/10.1038/ncb857
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb857
This article is cited by
-
Chemical fuels for molecular machinery
Nature Chemistry (2022)
-
Reversible catalysis
Nature Reviews Chemistry (2021)
-
A model of processive walking and slipping of kinesin-8 molecular motors
Scientific Reports (2021)
-
Experimental and theoretical energetics of walking molecular motors under fluctuating environments
Biophysical Reviews (2020)
-
Cargo adaptors regulate stepping and force generation of mammalian dynein–dynactin
Nature Chemical Biology (2019)