Abstract
The hierarchical organization of properly sized blood vessels ensures the correct distribution of blood to all organs of the body, and is controlled via haemodynamic cues. In current concepts, an endothelium-dependent shear stress set point causes blood vessel enlargement in response to higher flow rates, while lower flow would lead to blood vessel narrowing, thereby establishing homeostasis. We show that during zebrafish embryonic development increases in flow, after an initial expansion of blood vessel diameters, eventually lead to vessel contraction. This is mediated via endothelial cell shape changes. We identify the transforming growth factor beta co-receptor endoglin as an important player in this process. Endoglin mutant cells and blood vessels continue to enlarge in response to flow increases, thus exacerbating pre-existing embryonic arterial–venous shunts. Together, our data suggest that cell shape changes in response to biophysical cues act as an underlying principle allowing for the ordered patterning of tubular organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ochoa-Espinosa, A. & Affolter, M. Branching morphogenesis: from cells to organs and back. Cold Spring Harb. Perspect. Biol. 4, a008243 (2012).
Filosa, J. A., Morrison, H. W., Iddings, J. A., Du, W. & Kim, K. J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323, 96–109 (2016).
Joyner, M. J. & Casey, D. P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol. Rev. 95, 549–601 (2015).
Baeyens, N. & Schwartz, M. A. Biomechanics of vascular mechanosensation and remodeling. Mol. Biol. Cell 27, 7–11 (2016).
Kamiya, A. & Togawa, T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 239, H14–H21 (1980).
Langille, B. L. Arterial remodeling: relation to hemodynamics. Can. J. Physiol. Pharmacol. 74, 834–841 (1996).
Langille, B. L., Bendeck, M. P. & Keeley, F. W. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am. J. Physiol. 256, H931–H939 (1989).
Langille, B. L. & O’Donnell, F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231, 405–407 (1986).
Tuttle, J. L. et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am. J. Physiol. 281, H1380–H1389 (2001).
Baeyens, N. et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife 4, e04645 (2015).
Kamiya, A., Bukhari, R. & Togawa, T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull. Math. Biol. 46, 127–137 (1984).
Rodbard, S. Vascular caliber. Cardiology 60, 4–49 (1975).
Thoma, R. Untersuchungen über die Histogenese und Histomechanik des Gefässsystems (Verlag von Ferdinand Enke, 1893).
Pries, A. R., Hopfner, M., le Noble, F., Dewhirst, M. W. & Secomb, T. W. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat. Rev. Cancer 10, 587–593 (2010).
Tual-Chalot, S., Oh, S. P. & Arthur, H. M. Mouse models of hereditary hemorrhagic telangiectasia: recent advances and future challenges. Front. Genet. 6, 25 (2015).
Roman, B. L. et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129, 3009–3019 (2002).
Mahmoud, M. et al. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 106, 1425–1433 (2010).
Rochon, E. R., Menon, P. G. & Roman, B. L. Alk1 controls arterial endothelial cell migration in lumenized vessels. Development 143, 2593–2602 (2016).
Tual-Chalot, S. et al. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PLoS ONE 9, e98646 (2014).
Kamaid, A. et al. Betaglycan knock-down causes embryonic angiogenesis defects in zebrafish. Genesis 53, 583–603 (2015).
Lee, N. Y. et al. Endoglin regulates PI3-kinase/Akt trafficking and signaling to alter endothelial capillary stability during angiogenesis. Mol. Biol. Cell 23, 2412–2423 (2012).
Li, D. Y. et al. Defective angiogenesis in mice lacking endoglin. Science 284, 1534–1537 (1999).
Gougos, A. & Letarte, M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. Biol. Chem. 265, 8361–8364 (1990).
Seghers, L. et al. Shear induced collateral artery growth modulated by endoglin but not by ALK1. J. Cell Mol. Med. 16, 2440–2450 (2012).
Xu, C. et al. Arteries are formed by vein-derived endothelial tip cells. Nat. Commun. 5, 5758 (2014).
Childs, S., Chen, J. N., Garrity, D. M. & Fishman, M. C. Patterning of angiogenesis in the zebrafish embryo. Development 129, 973–982 (2002).
Chen, Q. et al. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol. 10, e1001374 (2012).
Kochhan, E. et al. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PLoS ONE 8, e75060 (2013).
Atri, D., Larrivee, B., Eichmann, A. & Simons, M. Endothelial signaling and the molecular basis of arteriovenous malformation. Cell Mol. Life Sci. 71, 867–883 (2014).
Bagatto, B. & Burggren, W. A three-dimensional functional assessment of heart and vessel development in the larva of the zebrafish (Danio rerio). Physiol. Biochem. Zool. 79, 194–201 (2006).
Malone, M. H. et al. Laser-scanning velocimetry: a confocal microscopy method for quantitative measurement of cardiovascular performance in zebrafish embryos and larvae. BMC Biotechnol. 7, 40 (2007).
Jamison, R. A., Samarage, C. R., Bryson-Richardson, R. J. & Fouras, A. In vivo wall shear measurements within the developing zebrafish heart. PLoS ONE 8, e75722 (2013).
Pappano, A. J. & Wier, W. G. Cardiovascular Physiology (Elsevier/Mosby, 2013).
Zhong, M. C., Wei, X. B., Zhou, J. H., Wang, Z. Q. & Li, Y. M. Trapping red blood cells in living animals using optical tweezers. Nat. Commun. 4, 1768 (2013).
Levesque, M. J. & Nerem, R. M. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 107, 341–347 (1985).
Boon, R. A. et al. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood 115, 2533–2542 (2010).
Nicoli, S. et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 464, 1196–1200 (2010).
Melchionna, R. et al. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 19, 629–631 (2005).
Hultin, S. et al. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat. Commun. 5, 3743 (2014).
Corti, P. et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138, 1573–1582 (2011).
Ong, A. C., Devuyst, O., Knebelmann, B. & Walz, G. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385, 1993–2002 (2015).
McDonald, J., Bayrak-Toydemir, P. & Pyeritz, R. E. Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet. Med. 13, 607–616 (2011).
Sigurbjornsdottir, S., Mathew, R. & Leptin, M. Molecular mechanisms of de novo lumen formation. Nat. Rev. Mol. Cell Biol. 15, 665–676 (2014).
Costantini, F. & Kopan, R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 (2010).
Forster, D. & Luschnig, S. Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila. Nat. Cell Biol. 14, 526–534 (2012).
Lubarsky, B. & Krasnow, M. A. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28 (2003).
Zuo, L., Iordanou, E., Chandran, R. R. & Jiang, L. Novel mechanisms of tube-size regulation revealed by the Drosophila trachea. Cell Tissue Res. 354, 343–354 (2013).
Dietrich, A. C., Lombardo, V. A., Veerkamp, J., Priller, F. & Abdelilah-Seyfried, S. Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev. Cell 30, 367–377 (2014).
Kim, C., Ye, F. & Ginsberg, M. H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 (2011).
Munjal, A. & Lecuit, T. Actomyosin networks and tissue morphogenesis. Development 141, 1789–1793 (2014).
Tzima, E., del Pozo, M. A., Shattil, S. J., Chien, S. & Schwartz, M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647 (2001).
Muenzner, P., Bachmann, V., Zimmermann, W., Hentschel, J. & Hauck, C. R. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science 329, 1197–1201 (2010).
Tulis, D. A., Unthank, J. L. & Prewitt, R. L. Flow-induced arterial remodeling in rat mesenteric vasculature. Am. J. Physiol. 274, H874–H882 (1998).
Unthank, J. L., Nixon, J. C., Burkhart, H. M., Fath, S. W. & Dalsing, M. C. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am. J. Physiol. 271, H914–H923 (1996).
Jin, Y. et al. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. http://dx.doi.org/10.1038/ncb3534 (2017).
Whitesell, T. R. et al. An α-smooth muscle actin (acta2/αsma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 9, e90590 (2014).
Mancini, M. L. et al. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev. Dyn. 238, 2479–2493 (2009).
Westerfield, M. The Zebrafish Book (University of Oregon Press, 1993).
Allinson, K. R., Carvalho, R. L., van den Brink, S., Mummery, C. L. & Arthur, H. M. Generation of a floxed allele of the mouse Endoglin gene. Genesis 45, 391–395 (2007).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82 (2011).
Villefranc, J. A., Amigo, J. & Lawson, N. D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 (2007).
Bussmann, J. & Schulte-Merker, S. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development 138, 4327–4332 (2011).
Siekmann, A. F. & Lawson, N. D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 (2007).
Mori, N. & Chang, K. PIV toolbox in MATLAB-Getting Started with mPIV (2004).
Hove, J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003).
Anton, H. et al. Pulse propagation by a capacitive mechanism drives embryonic blood flow. Development 140, 4426–4434 (2013).
Fåhræus, R. & Lindqvist, T. The viscosity of the blood in narrow capillary tubes. Am. J. Physiol. 96, 562–568 (1931).
Goetz, J. G. et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep. 6, 799–808 (2014).
Taubin, G. Estimation of planar curves, surfaces, and nonplanar space-curves defined by implicit equations with applications to edge and range image segmentation. IEEE Trans. Pattern Anal. 13, 1115–1138 (1991).
Eberly, D. H. 3D Game Engine Design: A Practical Approach to Real-time Computer Graphics 2nd edn (Morgan Kaufmann Publishers, 2007).
Acknowledgements
This work was funded by the Max Planck Society (A.F.S.), the Deutsche Forschungsgemeinschaft (DFG SI-1374/3-2; DFG SI-1374/4-1; DFG SI-1374/5-1; A.F.S.), and a European Research Council (ERC) starting grant (260794-ZebrafishAngio; A.F.S.). This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Cells-in-Motion Cluster of Excellence (EXC 1003-CIM), University of Münster, Germany. J.B. was supported by a long-term EMBO post-doctoral fellowship. L.J. was supported by The Swedish Research Council, The Swedish Cancer Society and Karolinska Institutet. We thank H. Arthur for providing the Engflox/flox mice and R. Adams for the Cdh5(PAC)-CreERT2 mice.
Author information
Authors and Affiliations
Contributions
W.W.S. and A.F.S. designed and interpreted the experiments and wrote the manuscript. J.B. identified the zebrafish eng homologue and generated the Tg(klf2a:YFP)mu107 zebrafish. R.T. performed cell culture experiments. E.V.L. performed FACS sorting of zebrafish embryos and performed qPCR experiments. R.M. and C.D. performed particle velocimetry, the optical rail experiments and determined flow parameters. T.A.-W. performed zebrafish cell shape analysis. M.J.H. and W.H. generated Tg(fli1a:lifeactEGFP)mu240 zebrafish. Y.J. and L.J. determined cell shape changes in eng mutant mice.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
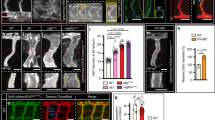
Supplementary Figure 1 Identification of a zebrafish endoglin orthologue.
(a) Schematic representation of the endoglin locus showing conservation of gene order (synteny) between human chromosome 9q33-34 and two paralogous loci on zebrafish chromosome 6 containing the Pregnancy-associated plasma protein A (pappa), Astrotactin 2 (astn2), a retrotransposed Atrial myosin light chain (myl4-RT), Deleted in bladder cancer 1 (dbc1), endoglin (eng), adenylate kinase 1 (ak1) and G-protein signaling modulator 1 (gpsm1) genes. Paralogous genes that were likely duplicated during the teleost whole genome duplication (WGD) event are indicated in blue. Subsequent gene loss on the paralogous chromosomes (lost genes in grey) most likely resulted in the current gene order. Pink shading indicates a small synteny block containing the Endoglin and AK1 genes. Dashed lines indicate synteny breaks between human and zebrafish. (b) Alignment of the conserved C-terminal region of zebrafish and human Endoglin. Asterisks indicate conserved residues. (c) Phylogenetic tree based on the alignment of the C-terminal sequences (transmembrane region and C-terminal domain) of vertebrate Tgfbr3 (green shading) and Endoglin (red shading). Numbers at branchpoints represent bootstrap values (percentage out of 1000 replicates). (d) Whole-mount in situ hybridization of eng shows vascular-restricted expression in 52 hpf zebrafish embryos. Expression is detected in the basal communicating artery (BCA), the posterior communicating segments (PCS), basilar artery (BA), primordial hindbrain channels (PHBC) and central arteries (CtAs) of the hindbrain as well as the dorsal aorta (DA, arrows), posterior cardinal vein (PCV) and dorsal longitudinal anastomotic vessel (DLAV, arrowheads) of the trunk vasculature. Scale bar in overview picture is 500 μm, in high magnification images scale bar is 100 μm. Images are representative of 48 out of 49 embryos. (e) Whole-mount in situ hybridization of eng expression in WT embryos treated with 15 uM nifedipine from 48–52 hpf. Note that blood flow reduction does not affect hindbrain expression, but decreases eng mRNA levels in the DLAV (arrowheads). Scale bar in overview picture is 500 μm, in high magnification images scale bar is 100 um. Images are representative of 45 out of 46 embryos.
Supplementary Figure 2 eng mRNA is subject to nonsense-mediated decay in engmu130 mutants.
(a) qPCR to detect zebrafish eng in FACS-sorted arterial and venous ECs from pooled 72 hpf embryos. Data is shown as relative expression normalized to WT arterial EC levels. In WTs, eng transcript can be detected at similar levels in the arterial and venous populations, and is almost non-detectable in engmu130 mutant ECs. Experiments were performed n = 3 times independently and were analysed by paired Student’s t-test. (b) Whole-mount in situ hybridization for endoglin expression at 32 hpf in embryos from WT, engmu130 and engmu132 heterozygous incrosses. All embryos carrying engmu132 alleles (which contain a 7nt substitution that does not introduce a frameshift) show the same staining intensity as WT, with particularly strong expression in the PCV (arrowhead). engmu130 +/− embryos already show decreased staining, which disappears almost entirely from the head and trunk vasculature in engmu130 mutants (arrows). Scale bar is 500 um. Images are representative of the number of embryos as indicated in the respective panel. NS, not significant, ∗P < 0.05,∗∗P < 0.01, ∗∗∗P < 0.001, error bars indicate s.e.m.
Supplementary Figure 3 ISV remodelling in WT and engmu130 17 dpf juveniles.
(a,b) Maximum intensity projections of confocal z-stacks of 17 dpf WT (a) and engmu130 mutants (b). Yellow arrowheads point to remodelled ISVs that no longer maintain connection to the DLAV. Scale bar is 100 μm. (c–f) Quantification of aISV identity (c), vISV identity (d), ISVs containing DsRed + erythrocytes (e) and remodelled ISVs (f). WTs and engmu130 mutants have no difference in ISV distribution or presence of RBCs, but engmu130 mutants show increased remodelling (n = 15 siblings (158 ISVs analysed) and n = 12 muts (122 ISVs analysed)). Analysed by unpaired Student’s t-test. NS, not significant, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, error bars indicate s.e.m.
Supplementary Figure 4 Arterial-venous specification is unaffected in endoglin mutants.
(a,b) Arterial-venous identity of ISVs as determined by flow direction and/or morphology (connection to DA or PCV) in WTs and engmu130 mutants. WTs and mutants have similar distribution of arteries and veins, with a slight increase in the number of unresolved ISV connections in engmu130 mutants. Indicated numbers of ISVs from 9 WT and 12 mut embryos were analysed. (c) Maximum intensity projection of confocal z-stack of engmu130 mutants at 72 hpf. Tg(fli1a:eng-GFP)mu156 embryos exhibit vascular-specific GFP expression, evidenced by overlap with Tg(kdrl:Hsa.HRAS-mCherry)s916. Scale bar is 100 μm. (d,e) Quantification of DA (d) and PCV (e) diameters in engmu130 mutants with or without the Tg(fli1a:eng-GFP)mu156 rescue construct. Tagged endoglin-GFP modestly rescues DA, but not PCV diameters (n = 12 GFP-negative, n = 13 GFP-positive engmu130 mutants). Analysed by unpaired Student’s t-test. (f) Quantification of the number of ISVs actively carrying RBCs shows rescue in engmu130 embryos carrying the Tg(fli1a:eng-GFP)mu156 rescue construct. 683 ISVs from 13 GFP-positive engmu130 mut embryos were analysed. NS, not significant, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, error bars indicate s.e.m.
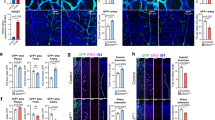
Supplementary Figure 5 Generation of the Tg(klf2a:YFP)mu107 reporter line.
(a) Schematic of the cloning strategy. A Citrine cassette was inserted behind the endogenous ATG of the klf2a gene contained in the CH73-180A21 BAC by homologous recombination. (b,c) Tg(klf2a:YFP)mu107 embryos display vascular-specific YFP expression, evidenced by overlap with Tg(kdrl:Hsa.HRAS-mCherry)s916. Embryos treated with tnnt2a MO lose YFP expression in the DA and PCV. Scale bar is 100 μm. (d–f) Quantification of YFP fluorescence intensity in the DA, PCV and ISVs in ctr MO and tnnt2a MO injected embryos (DA/PCV: n = 15 control MO, n = 15 tnnt2a MO; ISVs: n = 126 ISVs from 15 control MO, n = 133 ISVs from 15 tnnt2a MO). Analysed by Mann-Whitney U test. NS, not significant, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, error bars indicate s.e.m.
Supplementary Figure 6 Transplantation analysis of mosaic ISVs.
(a–c) Maximum intensity projections of confocal z-stack of mosaic ISVs from (a) WT- > WT, (b) Mut- > WT and (c) WT- > Mut transplantations. Optical sections reveal portions of lumen comprised only of donor cells, only of host cells, or a mosaic combination of host and donor cells. Scale bar on overview is 50 um, scale bar on lumen cross-section is 10 μm. (d-f) Quantification of mosaic ISV diameters depending on cellular composition from (d) WT- > WT, (e) Mut- > WT and (f) WT- > Mut transplantations. All ISVs from WT- > WT transplantations had normal diameters (d), while mutant cells caused dilation when they formed part or all of the lumen in Mut- > WT situations (e). WT cells were able to reduce ISV diameter when they formed all of the lumen in WT- > Mut scenarios (f). (WT- > WT n = 216 vessel segments from 54 ISVs; Mut- > WT n = 44 vessel segments from 11 ISVs; WT- > Mutn = 204 vessel segments from 51 ISVs). Analysed by One-Way ANOVA. (g) Schematic summarizing the requirement for eng function in every cell of a vascular tube to maintain proper dimensions. NS, not significant, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, error bars indicate s.e.m.
Supplementary Figure 7 Model for endoglin’s role in the biphasic response of ECs to blood flow.
(a) By 24 hpf, the first artery and vein have assembled by vasculogenesis. Blood flow starts. (b) As development proceeds, cardiac output rises. The axial vessels increase in diameter and carry an increasing volume of blood. At 48 hpf, the capillaries connecting the artery and vein have just begun to lumenize and carry flow. (c) Between 48 and 72 hpf, blood flow triggers shape changes in WT ECs. They elongate and align in the direction of flow, without changing their surface areas. This lengthens the vessel and reduces its diameter. The arterial constriction redirects blood flow through the capillary network. (d) In eng-deficient blood vessels, ECs are unable to limit their surface areas in response to blood flow. Although elongation still occurs to some extent, the increase in size impedes the ability of ECs to align to the flow. Ultimately, this results in vessel dilation and the formation of an arterio-venous shunt. The high velocities achieved in the shunt prevent RBCs from passing into capillaries, and the pulsatile quality of arterial flow is transmitted to the venous return.
Supplementary Figure 8
Original scanned blots used in Fig. 5e.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2443 kb)
Distal blood flow in WT fin regenerate at 5 dpa.
Brightfield movie of blood flow in the distal regenerating fin of WT fish at 5 dpa. Numbers label individual bony rays. Arrows indicate flow direction, while dashed lines denote arteries (red) and veins (blue). Flow from a centrally located artery distributes through a plexus to two veins at the end of each ray. Note weak flow reversal in lateral vein of the first ray, arcing over an inactive artery. (MP4 19382 kb)
AVMs and disturbed flow in engmu130 fin regenerate at 5 dpa.
Brightfield movie of blood flow in the distal regenerating fin of engmu130 mutant at 5 dpa. Numbers label individual bony rays. Arrows indicate flow direction, while dashed lines denote arteries (red) and veins (blue). Note prominent AVMs in second ray from the left. In ray #3, more proximally located AVMs either stop or reverse blood flow in the veins, and the central artery is not active. Ray #4 has strong flow reversal in the left vein, arcing over an inactive central artery. Note bleedings at distal end of all rays. (MP4 25340 kb)
ISV flow in 72 hpf sibling embryo.
Brightfield movie of RBC flow in the trunk of sibling embryo at 72 hpf. Nearly all ISVs on both sides of the embryo carry RBC flow. (MP4 23761 kb)
ISV flow in 72 hpf engmu130 embryo.
Brightfield movie of RBC flow in the trunk of engmu130 embryo at 72 hpf. Note that only 1 or 2 ISVs have persistent RBC flow, while most have no or only very intermittent RBC flow. (MP4 28677 kb)
Optical rail restores RBC flow in capillaries of engmu130 mutants.
Brightfield movie of RBC flow in the trunk of engmu130 embryo at 72 hpf. Holographic optical tweezers (HOT) are focused at entrance to an aISV, but laser is turned off. About halfway through movie the laser is turned on (red circle indicates HOT focal point), slows down RBCs that pass through the laser, and diverts them from the main arterial blood flow into the capillary. (MP4 7546 kb)
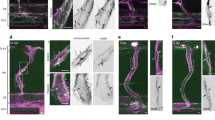
Time-lapse of cell shape changes in DA between 48 and 72 hpf in sibling embryo.
Maximum intensity projection of confocal z-stacks of Tg(fli1a:lifeactEGFP)mu240; Tg(fli1a:nEGFP)y7, and Tg(-0.8flt1:RFP)hu5333 in sibling embryo between 48 and 72 hpf. Acquisitions were made at 40 min intervals, shown at 5 frames per second. Note decrease in DA diameter between 48 and 72 hpf, and elongation of ECs. (MP4 1378 kb)
Time-lapse of cell shape changes in DA between 48 and 72 hpf in engmu130 embryo.
Maximum intensity projection of confocal z-stacks of Tg(fli1a:lifeactEGFP)mu240; Tg(fli1a:nEGFP)y7z, and Tg(-0.8flt1:RFP)hu5333 in engmu130 embryo between 48 and 72 hpf. Acquisitions were made at 40 min intervals, shown at 5 frames per second. Note a similar DA diameter at 48 hpf as sibling in Supplementary Video 4 that fails to decrease appreciably by 72 hpf. Note, additionally, the dramatic increase in EC sizes compared to siblings during the movie. (MP4 1437 kb)
Rights and permissions
About this article
Cite this article
Sugden, W., Meissner, R., Aegerter-Wilmsen, T. et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat Cell Biol 19, 653–665 (2017). https://doi.org/10.1038/ncb3528
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3528
This article is cited by
-
Hyperoxia prevents the dynamic neonatal increases in lung mesenchymal cell diversity
Scientific Reports (2024)
-
Role of endothelial PDGFB in arterio-venous malformations pathogenesis
Angiogenesis (2024)
-
A 96-wells fluidic system for high-throughput screenings under laminar high wall shear stress conditions
Microsystems & Nanoengineering (2023)
-
Mechanisms of endothelial flow sensing
Nature Cardiovascular Research (2023)
-
BMP10 functions independently from BMP9 for the development of a proper arteriovenous network
Angiogenesis (2023)