Abstract
The cytoplasmic polyadenylation element-binding (CPEB) proteins regulate pre-mRNA processing and translation of CPE-containing mRNAs in early embryonic development and synaptic activity. However, specific functions in adult organisms are poorly understood. Here we show that CPEB4 is required for adaptation to high-fat-diet- and ageing-induced endoplasmic reticulum (ER) stress, and subsequent hepatosteatosis. Stress-activated liver CPEB4 expression is dual-mode regulated. First, Cpeb4 mRNA transcription is controlled by the circadian clock, and then its translation is regulated by the unfolded protein response (UPR) through upstream open reading frames within the 5′UTR. Thus, the CPEB4 protein is synthesized only following ER stress but the induction amplitude is circadian. In turn, CPEB4 activates a second wave of UPR translation required to maintain ER and mitochondrial homeostasis. Our results suggest that combined transcriptional and translational Cpeb4 regulation generates a ‘circadian mediator’, which coordinates hepatic UPR activity with periods of high ER-protein-folding demand. Accordingly, CPEB4 deficiency results in non-alcoholic fatty liver disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Wang, S. & Kaufman, R. J. How does protein misfolding in the endoplasmic reticulum affect lipid metabolism in the liver? Curr. Opin. Lipidol. 25, 125–132 (2014).
Wang, M. & Kaufman, R. J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335 (2016).
Bertolotti, A., Zhang, Y., Hendershot, L., Harding, H. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Wek, R. C., Jiang, H. Y. & Anthony, T. G. Coping with stress-eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 (2006).
Tabas, I. & Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 (2011).
Cretenet, G., Le Clech, M. & Gachon, F. Circadian clock-coordinated 12 h period rhythmic activation of the IRE1α pathway controls lipid metabolism in mouse liver. Cell Metab. 11, 47–57 (2010).
Fernandez-Miranda, G. & Mendez, R. The CPEB-family of proteins, translational control in senescence and cancer. Ageing Res. Rev. 11, 460–472 (2012).
Ivshina, M., Lasko, P. & Richter, J. D. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu. Rev. Cell Dev. Biol. 30, 393–415 (2014).
Afroz, T. et al. A fly trap mechanism provides sequence-specific RNA recognition by CPEB proteins. Genes Dev. 28, 1498–1514 (2014).
Wang, X. P. & Cooper, N. G. Comparative in silico analyses of cpeb1-4 with functional predictions. Bioinformatics Biol. Insights 4, 61–83 (2010).
Mendez, R. et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302–307 (2000).
Pavlopoulos, E. et al. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell 147, 1369–1383 (2011).
Drisaldi, B. et al. SUMOylation is an inhibitory constraint that regulates the prion-like aggregation and activity of CPEB3. Cell Rep. 11, 1694–1702 (2015).
Igea, A. & Mendez, R. Meiosis requires a translational positive loop where CPEB1 ensues its replacement by CPEB4. EMBO J. 29, 2182–2193 (2010).
Guillén-Boixet, J. B. V., Salvatella, X. & Méndez, R. CPEB4 is regulated during cell cycle by ERK2/Cdk1-mediated phosphorylation and its assembly into liquid-like droplets. eLife 5, e19298 (2016).
Hake, L. E., Mendez, R. & Richter, J. D. Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol. Cell. Biol. 18, 685–693 (1998).
Tay, J. & Richter, J. D. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1, 201–213 (2001).
Hu, W., Yuan, B. & Lodish, H. F. Cpeb4-mediated translational regulatory circuitry controls terminal erythroid differentiation. Dev. Cell 30, 660–672 (2014).
Burns, D. M. & Richter, J. D. CPEB regulation of human cellular senescence, energy metabolism, and p53 mRNA translation. Genes Dev. 22, 3449–3460 (2008).
Ortiz-Zapater, E. et al. Key contribution of CPEB4-mediated translational control to cancer progression. Nat. Med. 18, 83–90 (2011).
Bava, F. A. et al. CPEB1 coordinates alternative 3’-UTR formation with translational regulation. Nature 495, 121–125 (2013).
Calderone, V. et al. Sequential functions of CPEB1 and CPEB4 regulate pathologic expression of VEGF and angiogenesis in chronic liver disease. Gastroenterology 150, 982–997 (2015).
Garcia-Pras, E. et al. Role and therapeutic potential of vascular stem/progenitor cells in pathological neovascularisation during chronic portal hypertension. Gut http://gut.bmj.com/content/early/2016/03/16/gutjnl-2015-311157 (2016).
Alexandrov, I. M. et al. Cytoplasmic polyadenylation element binding protein deficiency stimulates PTEN and Stat3 mRNA translation and induces hepatic insulin resistance. PLoS Genet. 8, e1002457 (2012).
Pique, M., Lopez, J. M., Foissac, S., Guigo, R. & Mendez, R. A combinatorial code for CPE-mediated translational control. Cell 132, 434–448 (2008).
Belloc, E. & Mendez, R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452, 1017–1021 (2008).
Kojima, S., Sher-Chen, E. L. & Green, C. B. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev. 26, 2724–2736 (2012).
Postic, C. & Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118, 829–838 (2008).
Browning, J. D. & Horton, J. D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 (2004).
Fabbrini, E., Sullivan, S. & Klein, S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010).
Deng, J. et al. Lipolysis response to endoplasmic reticulum stress in adipose cells. J. Biol. Chem. 287, 6240–6249 (2012).
Kan, M. C. et al. CPEB4 is a cell survival protein retained in the nucleus upon ischemia or endoplasmic reticulum calcium depletion. Mol. Cell. Biol. 30, 5658–5671 (2010).
Weill, L., Belloc, E., Bava, F. A. & Mendez, R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat. Struct. Mol. Biol. 19, 577–585 (2012).
Rutkowski, D. T. et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell 15, 829–840 (2008).
Raabe, M. et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103, 1287–1298 (1999).
Rao, M. S. & Reddy, J. K. Peroxisomal β-oxidation and steatohepatitis. Semin. Liver Dis. 21, 43–55 (2001).
Ota, T., Gayet, C. & Ginsberg, H. N. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 118, 316–332 (2008).
Volmer, R. & Ron, D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Biol. 33, 67–73 (2015).
Sagara, Y. & Inesi, G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 266, 13503–13506 (1991).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Gao, X. et al. Quantitative profiling of initiating ribosomes in vivo. Nat. Methods 12, 147–153 (2015).
Reid, D. W., Chen, Q., Tay, A. S., Shenolikar, S. & Nicchitta, C. V. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell 158, 1362–1374 (2014).
Giangarra, V., Igea, A., Castellazzi, C. L., Bava, F. A. & Mendez, R. Global analysis of CPEBs reveals sequential and non-redundant functions in mitotic cell cycle. PLoS ONE 10, e0138794 (2015).
Wethmar, K. et al. Comprehensive translational control of tyrosine kinase expression by upstream open reading frames. Oncogene 35, 1736–1742 (2016).
Kojima, S., Gendreau, K. L., Sher-Chen, E. L., Gao, P. & Green, C. B. Changes in poly(A) tail length dynamics from the loss of the circadian deadenylase Nocturnin. Sci. Rep. 5, 17059 (2015).
Zhang, Y. et al. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 348, 1488–1492 (2015).
Vollmers, C. et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl Acad. Sci. USA 106, 21453–21458 (2009).
Mauvoisin, D. et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl Acad. Sci. USA 111, 167–172 (2014).
Jouffe, C. et al. Perturbed rhythmic activation of signaling pathways in mice deficient for Sterol Carrier Protein 2-dependent diurnal lipid transport and metabolism. Sci. Rep. 6, 24631 (2016).
Kaufman, R. J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110, 1389–1398 (2002).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Kondratova, A. A. & Kondratov, R. V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13, 325–335 (2012).
Michelotti, G. A., Machado, M. V. & Diehl, A. M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10, 656–665 (2013).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
van Galen, P. et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 510, 268–272 (2014).
Mohrin, M. et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377 (2015).
Wang, L., Zeng, X., Ryoo, H. D. & Jasper, H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 10, e1004568 (2014).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Ortiz-Zapater, E. et al. Key contribution of CPEB4-mediated translational control to cancer progression. Nat. Med. 18, 83–90 (2012).
Calderone, V. et al. Sequential functions of CPEB1 and CPEB4 regulate pathologic expression of vascular endothelial growth factor and angiogenesis in chronic liver disease. Gastroenterology 150, 982–997.e930 (2016).
Hogan, B. Manipulating the Mouse Embryo: A Laboratory Manual 2nd edn (Cold Spring Harbor Laboratory Press, 1994).
Rutkowski, D. T. et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006).
Janicke, A., Vancuylenberg, J., Boag, P. R., Traven, A. & Beilharz, T. H. ePAT: a simple method to tag adenylated RNA to measure poly(A)-tail length and other 3′ RACE applications. RNA 18, 1289–1295 (2012).
Salmon, D. M. & Flatt, J. P. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int. J. Obes. 9, 443–449 (1985).
Planet, E., Attolini, C. S., Reina, O., Flores, O. & Rossell, D. htSeqTools: high-throughput sequencing quality control, processing and visualization in R. Bioinformatics 28, 589–590 (2012).
Smedley, D. et al. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 43, W589–W598 (2015).
uORFdb (accessed April 2016); http://www.compgen.uni-muenster.de/tools/uorfdb/index.hbi?
Acknowledgements
We thank the Advance Digital Microscopy, Biostatistics/Bioinformatics, Histopathology, Mouse Mutant, and Functional Genomics facilities at IRB Barcelona. We also thank S. Aznar-Benitah, J. Guinovart and members of R.M.’s laboratory for useful discussion and T. Yates for correcting the manuscript. This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO, BFU2011-30121, BIO2012-31043, BFU2014-54122-P, Consolider RNAREG CSD2009-00080, SAF2014-55473-R), the European Union FEDER funds, the Fundación Botín by the Banco Santander through its Santander Universities Global Division, the Scientific Foundation of the Spanish Association Against Cancer (AECC), and the Worldwide Cancer Research Foundation. C.M. held a ‘la Caixa’ predoctoral fellowship. IRB Barcelona is the recipient of a Severo Ochoa Award of Excellence from MINECO (Government of Spain). CIBER is an initiative from the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Contributions
C.M. performed all the studies and contributed to experimental design, data analysis and interpretation, and manuscript and figure preparation. R.M. and M.F. conceived and directed the study. A.Z., M.F. and R.M. wrote the manuscript and discussed the study. J.M. contributed to in vivo mouse experiments. D.S., M.H.-A. and M.G.-R. provided technical and conceptual assistance for experiments in Fig. 2 and Supplementary Fig. 1. O.R. performed bioinformatic analysis of Figs 3 and 7.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
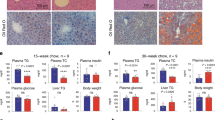
Supplementary Figure 1 (associated to Fig. 1). Cpeb4 gene-targeted mice develop liver steatosis.
(a) Immunoblot displaying CPEB4 and α-Tubulin protein levels in Cpeb4+/+, Cpeb4+/− and Cpeb4−/− liver extracts. Unprocessed original scans of blots are shown in Supplementary Fig. 7g. (b) CPEB4 immunohistochemistry in WT and Cpeb4KO liver sections. Scale bar, 100 μm. (c) Cpeb4 mRNA expression, normalized to TBP transcript levels, in livers from WT (n = 8) and Cpeb4KO (n = 8) mice. Two-sided Student’s t test, ∗∗∗P = 0.0001. (d) Changes in body weight of WT (n = 34) and Cpeb4KO (n = 32) mice fed standard diet. Two-way ANOVA, P = 0.3623. (e) Fed and overnight-fasted plasma glucose levels of mice fed HFD. WT-Fed, n = 8; WT-Fasted, n = 16; Cpeb4KO-Fed, n = 8; Cpeb4KO-Fasted, n = 14 mice. Two-way ANOVA, ∗P = 0.001. (f) Changes in body weight of WT (n = 40) and Cpeb4KO (n = 28) mice fed HFD. Two-way ANOVA, ∗P = 0.001. (g) Body weight of WT (n = 16) and Cpeb4KO (n = 10) mice aged for 80 weeks. Two-sided Student’s t test, ∗P = 0.0191. (h–j) 24-hour time course of RER (h), EE (i) and locomotor activity (j) of mice fed standard diet; WT, n = 12; Cpeb4KO, n = 12 mice. Two-way ANOVA. (k) Food intake (g/day) of WT (n = 8) and Cpeb4KO (n = 8) mice on HFD during 4 consecutive days. Two-way ANOVA, P = 0.7026. (l) Water intake in a 24-h period of WT (n = 12) and Cpeb4KO (n = 12) mice fed standard diet. Two-sided Student’s t test, P = 0.6823. (m) Plasma glucose levels of WT and Cpeb4KO fed, 6-h, and 24-h-fasted mice. WT-Fed, n = 20; WT-6hFasted, n = 22; WT-24hFasted, n = 22; Cpeb4KO-Fed, n = 20; Cpeb4KO-6hFasted, n = 20 mice; Cpeb4KO-24hFasted, n = 20 mice. Two-way ANOVA, P = 0.5986. (n–o) Fed and overnight fasted plasma insulin levels (n) and free fatty acid (FFA) plasma levels (o). Panel n: WT-Fed, n = 16; WT-Fasted, n = 18; Cpeb4KO-Fed, n = 18; Cpeb4KO-Fasted, n = 16 mice. Panel o: WT-Fed, n = 16; WT-Fasted, n = 18; Cpeb4KO-Fed, n = 18; Cpeb4KO-Fasted, n = 16 mice. Two-way ANOVA, P = 0.3629 (n), P = 0.6282 (o). (p–q) Glucose levels (p) and insulin levels (q) during glucose tolerance test in WT and Cpeb4KO mice. Panel p: WT, n = 18; Cpeb4KO, n = 18 mice. Panel q: WT, n = 12; Cpeb4KO, n = 12 mice. Two-way ANOVA, P = 0.2920 (p),P = 0.4257 (q). (r) Glucagon tolerance test after 6 h fasting in WT (n = 18) and Cpeb4KO (n = 18) mice. Two-way ANOVA, P = 0.0541. s,Glucose produced by primary hepatocytes in culture after treatment for 4 h with vehicle (dimethyl sulfoxide, DMSO), with a combination of 10 μM forskolin, 20 mM lactate, and 2 mM pyruvate (FSK) or with a combination of 300 μM dibutyryl-cAMP and 100 nM dexamethasone (cAMP); n = 12 primary hepatocyte cell lines from independent animals. Two-way ANOVA, P = 0.8810. For c–s, data are mean ± s.e.m. Experiments were replicated two (c–g,k–o,q), three (p,r) or four (h–j,s) times from biologically independent samples with similar results.
Supplementary Figure 2 (associated to Fig. 2). Cpeb4 deletion causes mitochondrial dysfunction and defective lipid metabolism in hepatocytes.
(a) Cpeb4 mRNA expression in livers from WT (n = 8) and Cpeb4LKO (n = 8) mice. Two-sided Student’s t test, P = 0.026. (b) Immunoblot displaying CPEB4 and α-Tubulin protein levels in WT and Cpeb4LKO mice. Unprocessed original scans of blots are shown in Supplementary Fig. 7h. (c) Weight evolution of WT (n = 22) and Cpeb4LKO (n = 30) mice fed standard diet. Two-way ANOVA, P = 0.8032. (d) Glucose tolerance test after overnight fasting in WT (n = 16) and Cpeb4LKO (n = 16) mice. Two-way ANOVA, P = 0.2175. (e) Plasma alanine aminotransferase levels of WT (n = 12) and Cpeb4LKO (n = 12) mice fed standard diet. Two-sided Student’s t test, P = 0.6622. f–g, Liver weight (f) and hepatic triglyceride content (g) of WT and Cpeb4LKO mice fed HFD. Panel f: WT, n = 44; Cpeb4LKO, n = 44 mice. Panel g: WT-CHOW, n = 12; WT-HFD, n = 18; Cpeb4LKO-CHOW, n = 20; Cpeb4LKO-HFD, n = 18 mice. Two-way ANOVA, ∗∗P = 0.0212 (f) ∗∗∗P = 0.017 (g). (h) Photograph of the liver, and H&E and Oil Red O staining of liver sections from the same animals. Representative images of 20 independent experiments are shown. Scale bar, 100 μm. (i) Growth curve of WT (n = 44) and Cpeb4LKO (n = 48) mice on HFD. Two-way ANOVA, P = 0.2922. (j) Fasn and Scd1 gene expression analysis by qRT-PCR of livers from WT (n = 16) or Cpeb4LKO (n = 16) mice. Two-way ANOVA, P = 0.4274. (k) Analysis of palmitate uptake in primary hepatocytes; n = 18 biologically independent dishes per group. Two-sided Student’s t test, P = 0.9654. (l) Immunoblot for the indicated mitochondrial markers and loading controls in WT and Cpeb4KO liver extracts, n = 3 biologically independent samples. Unprocessed original scans of blots are shown in Supplementary Fig. 7i. (m) mtDNA quantification normalized to nuclear DNA content by qRT-PCR of livers from WT (n = 16) and Cpeb4KO (n = 16) mice. Two-sided Student’s t test, P = 0.750. For a,c–g,i–k and m, data are mean ± s.e.m. Experiments were replicated two (a,c–e,g,j), three (k) or four (f,i) times from biologically independent samples with similar results.
Supplementary Figure 3 (associated to Fig. 4). CPEB4 depletion leads to defective adaptation to chronic ER-stress.
(a) Apoptosis analysis of WT and Cpeb4KO MEFs measured by flow cytometry as the percentage of annexin V-positive cells after treatment with H202 (100 μM) or ionizing radiation (IR) (5 Gy) for 24 h; n = 4 biologically independent MEF cell lines. Two-way ANOVA, P = 0.9775. (b) Left: TUNEL staining of liver sections of WT and Cpeb4LKO mice injected with 1 mg kg−1 TM and killed 48 h later. Scale bar, 100 μm. Arrows indicate apoptotic cells. Right: Quantification of the number of apoptotic cells in livers from WT (n = 20) and Cpeb4LKO (n = 20) mice. Two-sided Student’s t test, ∗P = 0.0216. Data are mean ± s.e.m. Experiments in a,b were replicated two times from biologically independent samples with similar results.
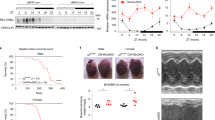
Supplementary Figure 4 (associated to Figs 5 and 6). CPEB4 synthesis and translation of CPE-regulated mRNAs are upregulated by UPR.
(a) Atf4 mRNA analysis in WT MEFs treated with 1 μM thapsigargin (TG) for the indicated times. (b) qRT-PCR expression analysis of the different luciferase constructs in HepG2 cells treated with 0.1 μM TG for 6 h; n = 18 biologically independent dishes. Two-way ANOVA, P = 0.6067. (c) Left: Immunoblot for the indicated proteins in WT or PerkKO MEFs treated with 1.5 μM TG and harvested at the indicated times. Right: Immunoblot quantification. Unprocessed original scans of blots are shown in Supplementary Fig. 7j. (d) Total translation of Txnip assessed by ribosome profiling in MEFs treated with 1 μM TG for the indicated times (Reid D.W. et al., 2014). (e) Txnip 3′UTR sequence in various mammalian species. Conserved CPE-elements are highlighted. (f) Left: Txnip mRNA poly(A) tail length quantification by ePAT assay in WT and Cpeb4KO MEFs treated with TG for 2 h. Right: Quantification of the area under the curve (AU); n = 8 biologically independent MEF cell lines. Two-way ANOVA, ∗P = 0.0105. Data are mean ± s.e.m. in a,c,f and mean ± s.d. in b. Experiments were replicated two (c,f) or three (b) times from biologically independent samples with similar results.
Supplementary Figure 5 (associated to Fig. 7). uORFs and CPEs determine mRNA activation kinetics, which is influenced by the circadian clock.
(a) Gene expression analysis by qRT-PCR of Bmal1 and Per2 in WT and Cpeb4KO mouse livers at the indicated ZT. Two-way ANOVA, P = 0.95. (b) Cpeb4 mRNA levels in livers of WT fed mice at the indicated ZTs.
Supplementary Figure 6 Working model: sequential waves of translational activation during ER-stress mediated by PERK/uORFs and CPEB4/CPEs.
The UPR triggers general translation inhibition. However, mRNAs harbouring uORFs in their 5′UTRs are translationally activated at early time points after ER-stress, including Cpeb4 mRNA. When CPEB4 is produced, it activates the translation of CPE-containing mRNAs at late time points generating a second wave of protein production.
Supplementary Figure 7 Unprocessed originals scans of blots.
(a) Western blot corresponding to Fig. 3a. (b) Western blot corresponding to Fig. 3e. (c) Western blot corresponding to Fig. 5a. (d) Western blot corresponding to Fig. 6b. (e) Western blot corresponding to Fig. 7d. (f) Western blot corresponding to Fig. 7e. (g) Western blot corresponding to Supplementary Fig. 1a. (h) Western blot corresponding to Supplementary Fig. 2b. (i) Western blot corresponding to Supplementary Fig. 2l. (j) Western blot corresponding to Supplementary Fig. 4c.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5681 kb)
Supplementary Table 1
Supplementary Information (XLSX 36 kb)
Supplementary Table 2
Supplementary Information (XLSX 12 kb)
Supplementary Table 3
Supplementary Information (XLSX 9 kb)
Supplementary Table 4
Supplementary Information (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Maillo, C., Martín, J., Sebastián, D. et al. Circadian- and UPR-dependent control of CPEB4 mediates a translational response to counteract hepatic steatosis under ER stress. Nat Cell Biol 19, 94–105 (2017). https://doi.org/10.1038/ncb3461
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3461
This article is cited by
-
Comparative analyses of vertebrate CPEB proteins define two subfamilies with coordinated yet distinct functions in post-transcriptional gene regulation
Genome Biology (2022)
-
Control of immediate early gene expression by CPEB4-repressor complex-mediated mRNA degradation
Genome Biology (2022)
-
The role of CPEB family proteins in the nervous system function in the norm and pathology
Cell & Bioscience (2021)
-
Genetic loss-of-function of activating transcription factor 3 but not C-type lectin member 5A prevents diabetic peripheral neuropathy
Laboratory Investigation (2021)
-
CPEB3-mediated MTDH mRNA translational suppression restrains hepatocellular carcinoma progression
Cell Death & Disease (2020)