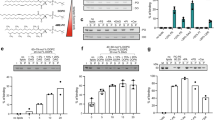
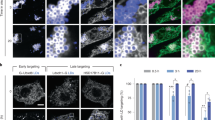
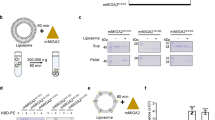
Abstract
Lipid droplets (LDs) are endoplasmic reticulum (ER)-derived lipid storage organelles uniquely encapsulated by phospholipid monolayers. LD membrane proteins are embedded into the monolayer in a monotopic hairpin topology and are therefore likely to have requirements for their biogenesis distinct from those inserting as bitopic and polytopic proteins into phospholipid bilayers. UBXD8 belongs to a subfamily of hairpin proteins that localize to both the ER and LDs, and are initially inserted into the cytoplasmic leaflet of the ER bilayer before partitioning to the LD monolayer. The molecular machinery responsible for inserting hairpin proteins into membranes, however, is unknown. Here, we report that newly synthesized UBXD8 is post-translationally inserted into discrete ER subdomains by a mechanism requiring cytosolic PEX19 and membrane-integrated PEX3, proteins hitherto exclusively implicated in peroxisome biogenesis. Farnesylation of PEX19 uncouples ER/LD and peroxisome targeting, expanding the function of this peroxin to an ER-targeting pathway and suggesting a coordinated biogenesis of LDs and peroxisomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Walther, T. C. & Farese, R. V. Jr Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81, 687–714 (2012).
Thiele, C. & Spandl, J. Cell biology of lipid droplets. Curr. Opin. Cell Biol. 20, 378–385 (2008).
Stevanovic, A. & Thiele, C. Monotopic topology is required for lipid droplet targeting of ancient ubiquitous protein 1. J. Lipid Res. 54, 503–513 (2013).
Klemm, E. J., Spooner, E. & Ploegh, H. L. Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control. J. Biol. Chem. 286, 37602–37614 (2011).
Wilfling, F. et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 24, 384–399 (2013).
Zehmer, J. K. et al. Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J. Cell Sci. 122, 3694–3702 (2009).
Olzmann, J. A., Richter, C. M. & Kopito, R. R. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl Acad. Sci. USA 110, 1345–1350 (2013).
Keenan, R. J., Freymann, D. M., Stroud, R. M. & Walter, P. The signal recognition particle. Annu. Rev. Biochem. 70, 755–775 (2001).
Rapoport, T. A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669 (2007).
Favaloro, V., Spasic, M., Schwappach, B. & Dobberstein, B. Distinct targeting pathways for the membrane insertion of tail-anchored (TA) proteins. J. Cell Sci. 121, 1832–1840 (2008).
Stefanovic, S. & Hegde, R. S. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell 128, 1147–1159 (2007).
Vilardi, F., Lorenz, H. & Dobberstein, B. WRB is the receptor for TRC40/Asna1-mediated insertion of tail-anchored proteins into the ER membrane. J. Cell Sci. 124, 1301–1307 (2011).
Yamamoto, Y. & Sakisaka, T. Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol. Cell 48, 387–397 (2012).
Mariappan, M. et al. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature 466, 1120–1124 (2010).
Lipp, J. & Dobberstein, B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J. Cell Biol. 102, 2169–2175 (1986).
Suzuki, M. et al. Derlin-1 and UBXD8 are engaged in dislocation and degradation of lipidated ApoB-100 at lipid droplets. Mol. Biol. Cell 23, 800–810 (2012).
Lee, J. N. et al. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc. Natl Acad. Sci. USA 107, 21424–21429 (2010).
Meyer, D. I. & Dobberstein, B. Identification and characterization of a membrane component essential for the translocation of nascent proteins across the membrane of the endoplasmic reticulum. J. Cell Biol. 87, 503–508 (1980).
Gilmore, R., Blobel, G. & Walter, P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J. Cell Biol. 95, 463–469 (1982).
Favaloro, V., Vilardi, F., Schlecht, R., Mayer, M. P. & Dobberstein, B. Asna1/TRC40-mediated membrane insertion of tail-anchored proteins. J. Cell Sci. 123, 1522–1530 (2010).
Brambillasca, S. et al. Transmembrane topogenesis of a tail-anchored protein is modulated by membrane lipid composition. EMBO J. 24, 2533–2542 (2005).
Schmidt, F. et al. Insights into peroxisome function from the structure of PEX3 in complex with a soluble fragment of PEX19. J. Biol. Chem. 285, 25410–25417 (2010).
Fang, Y., Morrell, J. C., Jones, J. M. & Gould, S. J. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J. Cell Biol. 164, 863–875 (2004).
Schmidt, F. et al. The role of conserved PEX3 regions in PEX19-binding and peroxisome biogenesis. Traffic 13, 1244–1260 (2012).
Aranovich, A., Hua, R., Rutenberg, A. D. & Kim, P. K. PEX16 contributes to peroxisome maintenance by constantly trafficking PEX3 via the ER. J. Cell Sci. 127, 3675–3686 (2014).
Toro, A. et al. Evaluation of the role of the endoplasmic reticulum-Golgi transit in the biogenesis of peroxisomal membrane proteins in wild type and peroxisome biogenesis mutant CHO cells. Biol. Res. 40, 231–249 (2007).
Mayerhofer, P. U., Bano-Polo, M., Mingarro, I. & Johnson, A. E. Human peroxin PEX3 is co-translationally integrated into the ER and exits the ER in budding vesicles. Traffic 17, 117–130 (2016).
Kim, P. K., Mullen, R. T., Schumann, U. & Lippincott-Schwartz, J. The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J. Cell Biol. 173, 521–532 (2006).
Geuze, H. J. et al. Involvement of the endoplasmic reticulum in peroxisome formation. Mol. Biol. Cell 14, 2900–2907 (2003).
Kim, P. K. & Hettema, E. H. Multiple pathways for protein transport to peroxisomes. J. Mol. Biol. 427, 1176–1190 (2015).
Vastiau, I. M. et al. Farnesylation of Pex19p is not essential for peroxisome biogenesis in yeast and mammalian cells. Cell. Mol. Life Sci. 63, 1686–1699 (2006).
Halbach, A. et al. Targeting of the tail-anchored peroxisomal membrane proteins PEX26 and PEX15 occurs through C-terminal PEX19-binding sites. J. Cell Sci. 119, 2508–2517 (2006).
Sacksteder, K. A. et al. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J. Cell Biol. 148, 931–944 (2000).
Schuldiner, M. et al. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 (2008).
Lee, J. G. & Ye, Y. Bag6/Bat3/Scythe: a novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation. Bioessays 35, 377–385 (2013).
Hessa, T. et al. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 (2011).
Rodrigo-Brenni, M. C., Gutierrez, E. & Hegde, R. S. Cytosolic quality control of mislocalized proteins requires RNF126 recruitment to Bag6. Mol. Cell 55, 227–237 (2014).
Agrawal, G. & Subramani, S. Emerging role of the endoplasmic reticulum in peroxisome biogenesis. Front. Physiol. 4, 286 (2013).
Hoepfner, D., Schildknegt, D., Braakman, I., Philippsen, P. & Tabak, H. F. Contribution of the endoplasmic reticulum to peroxisome formation. Cell 122, 85–95 (2005).
Agrawal, G., Fassas, S. N., Xia, Z. J. & Subramani, S. Distinct requirements for intra-ER sorting and budding of peroxisomal membrane proteins from the ER. J. Cell Biol. 212, 335–348 (2016).
Lodhi, I. J. & Semenkovich, C. F. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 19, 380–392 (2014).
Hajra, A. K. & Das, A. K. Lipid biosynthesis in peroxisomes. Ann. N Y Acad. Sci. 804, 129–141 (1996).
Bartz, R. et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48, 837–847 (2007).
Tabak, H. F., Murk, J. L., Braakman, I. & Geuze, H. J. Peroxisomes start their life in the endoplasmic reticulum. Traffic 4, 512–518 (2003).
Schrul, B., Kapp, K., Sinning, I. & Dobberstein, B. Signal peptide peptidase (SPP) assembles with substrates and misfolded membrane proteins into distinct oligomeric complexes. Biochem. J. 427, 523–534 (2010).
Leznicki, P. & High, S. SGTA antagonizes BAG6-mediated protein triage. Proc. Natl Acad. Sci. USA 109, 19214–19219 (2012).
Landry, J. J. et al. The genomic and transcriptomic landscape of a HeLa cell line. G3 (Bethesda) 3, 1213–1224 (2013).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Acknowledgements
We thank J. Elias and F. McAllister for protein identification by mass spectrometry, J. Perrino for gold enhancement, embedding and sectioning of the electron microscopy samples, and J. Mulholland for help with structured illumination microscopy. We are grateful to B. Dobberstein (ZMBH, University of Heidelberg, Germany), F. Vilardi (Institute of Molecular Biology, University of Göttingen, Göttingen, Germany), V. Favaloro (Department of Biology, Howard Hughes Medical Institute, Stanford University, Stanford, USA), S. High (Faculty of Life Sciences, University of Manchester, Manchester, UK) and G. Dodt (Interfaculty Institute of Biochemistry, University of Tübingen, Tübingen, Germany) for generously sharing valuable reagents, to S. B. Yamada for technical help, to M. Nachury for access to his fluorescence microscope, and to D. Mick and M. Pearce for critical reading of the manuscript. This work was supported by a National Institutes of Health grant GM074874 to R.R.K. and, in part, by the Shared Instrumentation Grants (SIG) 1S10OD01227601 and 1S10RR02678001.
Author information
Authors and Affiliations
Contributions
B.S. performed and analysed all experiments, prepared the figures and wrote the first draft of the manuscript. B.S. and R.R.K. jointly conceived the experimental design, interpreted the results and wrote subsequent drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 2 Characterization of Pre-treated RMs, Related to Figure 1.
Pre-treated rough microsomes (RMs) were analysed by SDS-PAGE and immunoblotting using anti-PDI, anti-calreticulin, anti-calnexin or anti-TRAM antibodies as indicated. T-RM: trypsin-treated RMs; NEM-RM: N-ethylmaleimide treated RMs. A representative blot from a single experiment is shown. The immunoblot analysis was repeated once with similar results. RMs were pre-treated in batch once and used for all experiments. Uncropped scans of all gels are available in Supplementary Figure 8.
Supplementary Figure 3 Analyses of UBXD8 Pre-Insertion Complex Components, Related to Figure 3.
(a) Sucrose density fractionation experiment of in vitro translated sUBXD8FLop performed in parallel with experiment in figure 3d. Here, UBXD8 complexes were S-affinity purified from individual fractions and analysed by SDS-PAGE and immunoblotting using anti-opsin (UBXD8), -BAG6 and -PEX19 antibodies. A representative blot from a single experiment is shown, the experiment was repeated once with similar results. (b) UBXD8, PEX19 and BAG6 amounts in the individual fractions of a were quantified by densitometry and the relative ratios of PEX19/UBXD8 or BAG6/UBXD8 calculated. A.U. arbitrary units. Uncropped scans of all gels are available in Supplementary Figure 8.
Supplementary Figure 4 Generation of PEX19−/− Cells by CRISPR/Cas9 Genome-Editing, Related to Figure 4.
(a) Schematic outline of the genome-editing strategy to delete endogenous PEX19 in HeLa cells. Exons 1 to 8 (E1-E8) are indicated. Selected guide RNAs 1, 2 and 3 target a sequence within exon 1, guide RNA 4 targets exon 2. PAM sequences are depicted in grey. (b) SDS-PAGE and immunoblot analysis of wild-type (WT) cells and genome-edited PEX19−/− clonal cell lines using indicated antibodies. A representative blot of a single experiment is shown, the experiment was repeated twice with similar results. Clonal cell lines used in the main figures are indicated in bold. (c) PEX19−/− cells lack peroxisomes. Immunofluorescence microscopy of WT and PEX19−/− cell lines using antibodies against catalase, a marker for mature peroxisomes. Representative images of a single experiment are shown, The experiment was repeated once with similar results. Scale bars, 10 μm. (d) PEX19 overexpression in PEX19−/− cell lines reverts mitochondrial mislocalisation phenotype of endogenous UBXD8. WT or PEX19−/− cell lines were transfected with plasmids encoding WT PEX19 and analysed by immunofluorescence microscopy using anti-PEX19 and anti-UBXD8 antibodies. Representative images of a single experiment are shown, the experiment was repeated twice with similar results. Open and white arrowheads indicate transfected and non-transfected cells, respectively. Scale bars, 10 μm. Uncropped scans of all gels are available in Supplementary Figure 8.
Supplementary Figure 5 Colocalisation analysis of sUBXD8FLop foci with endogenous PEX14, Related to Figure 6
(a) In vitro translated (IVT) sUBXD8FLop was imported into semi-permeabilised WT cells and visualized by IF/SIM microscopy using anti-opsin and anti-PEX14 antibodies. A representative single SIM z-slice inset (left) and a fluorescence intensity profile from indicated line scan (right) from a single experiment are shown to illustrate how colocalisation of sUBXD8FLop foci with PEX14-positive foci was quantified in b. A.U.: arbitrary units. (b) Quantification of the distance between sUBXD8FLop foci and PEX14-positive foci using line profiles of fluorescence intensity as shown in a. 53 sUBXD8FLop foci were analysed from one representative cell. The experiment itself was not repeated but independent SIM images were acquired from three individual cells and showed similar results. Grey-shaded area indicates sUBXD8FLop foci that colocalised with PEX14 foci and in addition were found adjacent to sUBXD8FLop-negative PEX14 foci within a distance of 50–250 nm.
Supplementary Figure 6 siRNA-mediated PEX5 knock-down in WT cells, Related to Figure 6.
WT cells were transfected with the indicated siRNAs for 120 h and analysed by either immunoblotting using the indicated antibodies to confirm reduction of endogenous PEX5 levels (a) or by IF microscopy using catalase-specific antibodies to indicate lack of mature peroxisomes (b). Representative images and blots from parallel experiments are shown. The experiments were repeated three times with similar results. NT: non-transfected. Scale bars, 10 μm. Asterisk indicates cross-reactive band. Uncropped scans are available in Supplementary Figure 8.
Supplementary Figure 7 Stable expression of PEX19C296S in PEX19−/− cells restores peroxisome biogenesis, Related to Figure 7.
IF microscopy of WT, PEX19−/−, and PEX19−/− PEX19C296S cells as indicated using catalase-specific antibodies. Scale bar, 10 μm. Representative images from a single experiment are shown. For WT cells, the experiment was repeated four times with similar results. For PEX19−/− cells nine independent clonal PEX19−/− cell lines were analysed in two independent experiments with similar results. For PEX19−/− PEX19C296S cells, three independent cell lines were analysed in two independent experiments with similar results.
Supplementary Figure 8 Model of HP-protein insertion into ER-subdomains as exemplified by UBXD8.
Newly synthesized HP-proteins are released from cytosolic ribosomes and engage either BAG6 or PEX19. We can envision three distinct roles for BAG6 binding in HP-protein biogenesis. First, BAG6 could function in a parallel ER-targeting pathway, competing with PEX19 for binding to the newly synthesized protein (a). However, the inability to detect efficient UBXD8 insertion into RMs when incubated with sucrose density gradient fractions containing BAG6 but not PEX19, and the mitochondrial mislocalisation of UBXD8 in Pex19−/− cells argue against a direct and PEX19-independent role for BAG6 in HP-protein membrane insertion. A second possibility (b) is that BAG6 and PEX19 bind to nascent HP-proteins sequentially in a manner reminiscent of the hand-off of TA proteins from BAG6 to TRC401. Finally (c), BAG6 could serve a “triage” role in its capacity of a quality control “holdase”2,3 directing non-inserted HP-proteins for degradation by the ubiquitin-proteasome system. These latter two models are not mutually exclusive and further investigation is required to assess their relative contributions to HP-protein biogenesis. The PEX19-HP-protein pre-insertion complex does not target clients to lipid droplets (LD) or peroxisomes but inserts them into ER-subdomains with the help of PEX3. PEX19 farnesylation ensures correct client protein insertion into ER-subdomains, whereas non-farnesylated PEX19 can promote mistargeting of a HP-protein to peroxisomes. TG: Triglycerides; FA: fatty acids.
Supplementary information
Supplementary Information
Supplementary Information (PDF 737 kb)
UBXD8 insertion sites align with the ER marker calnexin.
A full z-stack of the specimen shown in figure 2d is shown. In vitro translated sUBXD8FLop was imported into semi-permeabilised cells, which were examined by immunofluorescence microscopy using anti-opsin (green) and anti-calnexin (red) antibodies, respectively. (AVI 319 kb)
Rights and permissions
About this article
Cite this article
Schrul, B., Kopito, R. Peroxin-dependent targeting of a lipid-droplet-destined membrane protein to ER subdomains. Nat Cell Biol 18, 740–751 (2016). https://doi.org/10.1038/ncb3373
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3373
This article is cited by
-
Identification of two pathways mediating protein targeting from ER to lipid droplets
Nature Cell Biology (2022)
-
Mechanismen des Proteinimports in das humane endoplasmatische Retikulum
BIOspektrum (2022)
-
Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5
Nature Communications (2020)
-
Dynamics and functions of lipid droplets
Nature Reviews Molecular Cell Biology (2019)
-
Lipid droplet and peroxisome biogenesis occur at the same ER subdomains
Nature Communications (2018)