Abstract
Integrin receptor activation initiates the formation of integrin adhesion complexes (IACs) at the cell membrane that transduce adhesion-dependent signals to control a multitude of cellular functions. Proteomic analyses of isolated IACs have revealed an unanticipated molecular complexity; however, a global view of the consensus composition and dynamics of IACs is lacking. Here, we have integrated several IAC proteomes and generated a 2,412-protein integrin adhesome. Analysis of this data set reveals the functional diversity of proteins in IACs and establishes a consensus adhesome of 60 proteins. The consensus adhesome is likely to represent a core cell adhesion machinery, centred around four axes comprising ILK–PINCH–kindlin, FAK–paxillin, talin–vinculin and α-actinin–zyxin–VASP, and includes underappreciated IAC components such as Rsu-1 and caldesmon. Proteomic quantification of IAC assembly and disassembly detailed the compositional dynamics of the core cell adhesion machinery. The definition of this consensus view of integrin adhesome components provides a resource for the research community.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Liu, S., Calderwood, D. A. & Ginsberg, M. H. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563–3571 (2000).
Morse, E. M., Brahme, N. N. & Calderwood, D. A. Integrin cytoplasmic tail interactions. Biochemistry (Mosc.) 53, 810–820 (2014).
Winograd-Katz, S. E., Fässler, R., Geiger, B. & Legate, K. R. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 15, 273–288 (2014).
Zaidel-Bar, R. & Geiger, B. The switchable integrin adhesome. J. Cell Sci. 123, 1385–1388 (2010).
Zaidel-Bar, R., Itzkovitz, S., Ma’ayan, A., Iyengar, R. & Geiger, B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867 (2007).
Byron, A., Humphries, J. D., Bass, M. D., Knight, D. & Humphries, M. J. Proteomic analysis of integrin adhesion complexes. Sci. Signal. 4, pt2 (2011).
Byron, A., Humphries, J. D., Craig, S. E., Knight, D. & Humphries, M. J. Proteomic analysis of α4β1 integrin adhesion complexes reveals α-subunit-dependent protein recruitment. Proteomics 12, 2107–2114 (2012).
Byron, A. et al. A proteomic approach reveals integrin activation state-dependent control of microtubule cortical targeting. Nat. Commun. 6, 6135 (2015).
Huang, I.-H. et al. GEF-H1 controls focal adhesion signaling that regulates mesenchymal stem cell lineage commitment. J. Cell Sci. 127, 4186–4200 (2014).
Humphries, J. D. et al. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2, ra51 (2009).
Kuo, J.-C., Han, X., Hsiao, C.-T., Yates, J. R. & Waterman, C. M. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–393 (2011).
Ng, D. H. J., Humphries, J. D., Byron, A., Millon-Frémillon, A. & Humphries, M. J. Microtubule-dependent modulation of adhesion complex composition. PLoS ONE 9, e115213 (2014).
Robertson, J. et al. Defining the phospho-adhesome through the phosphoproteomic analysis of integrin signalling. Nat. Commun. 6, 6265 (2015).
Schiller, H. B., Friedel, C. C., Boulegue, C. & Fässler, R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 12, 259–266 (2011).
Schiller, H. B. et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625–636 (2013).
Yue, J. et al. Microtubules regulate focal adhesion dynamics through MAP4K4. Dev. Cell 31, 572–585 (2014).
Jones, M. C. et al. Isolation of integrin-based adhesion complexes. Curr. Protoc. Cell Biol. 66, 9.8.1–9.8.15 (2015).
Kuo, J.-C., Han, X., Yates, J. R. & Waterman, C. M. Isolation of focal adhesion proteins for biochemical and proteomic analysis. Methods Mol. Biol. 757, 297–323 (2012).
Humphries, J. D., Paul, N. R., Humphries, M. J. & Morgan, M. R. Emerging properties of adhesion complexes: what are they and what do they do? Trends Cell Biol. 25, 388–397 (2015).
Geiger, T. & Zaidel-Bar, R. Opening the floodgates: proteomics and the integrin adhesome. Curr. Opin. Cell Biol. 25, 562–568 (2012).
Smith, M. A., Hoffman, L. M. & Beckerle, M. C. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 24, 575–583 (2014).
Uemura, A., Nguyen, T.-N., Steele, A. N. & Yamada, S. The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. Biophys. J. 101, 1069–1075 (2011).
Schiller, H. B. & Fässler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519 (2013).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Janovick, J. A., Natarajan, K., Longo, F. & Conn, P. M. Caldesmon: a bifunctional (calmodulin and actin) binding protein which regulates stimulated gonadotropin release. Endocrinology 129, 68–74 (1991).
Manders, E. M. M., Verbeek, F. J. & Aten, J. A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 169, 375–382 (1993).
Dougherty, G. W., Jose, C., Gimona, M. & Cutler, M. L. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur. J. Cell Biol. 87, 721–734 (2008).
Attanasio, F. et al. Novel invadopodia components revealed by differential proteomics analysis. Eur. J. Cell Biol. 90, 115–127 (2011).
Cervero, P., Himmel, M., Krüger, M. & Linder, S. Proteomic analysis of podosome fractions from macrophages reveals similarities to spreading initiation centres. Eur. J. Cell Biol. 91, 908–922 (2012).
de Hoog, C. L., Foster, L. J. & Mann, M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell 117, 649–662 (2004).
Ezratty, E. J., Partridge, M. A. & Gundersen, G. G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581–590 (2005).
Beggs, A. H. et al. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J. Biol. Chem. 267, 9281–9288 (1992).
Bialkowska, K. et al. The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J. Biol. Chem. 285, 18640–18649 (2010).
Karaköse, E., Schiller, H. B. & Fässler, R. The kindlins at a glance. J. Cell Sci. 123, 2353–2356 (2010).
Zhang, Y. et al. Nuclear SIPA1 activates integrin β1 promoter and promotes invasion of breast cancer cells. Oncogene 34, 1451–1462 (2015).
Bai, S. W. et al. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 9, 54 (2011).
Buxbaum, A. R., Wu, B. & Singer, R. H. Single β-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science 343, 419–422 (2014).
Chicurel, M. E., Singer, R. H., Meyer, C. J. & Ingber, D. E. Integrin binding and mechanical tension induce movement of mRNA and ribosomes to focal adhesions. Nature 392, 730–733 (1998).
Katz, Z. B. et al. β-Actin mRNA compartmentalization enhances focal adhesion stability and directs cell migration. Genes Dev. 26, 1885–1890 (2012).
Willett, M., Pollard, H. J., Vlasak, M. & Morley, S. J. Localization of ribosomes and translation initiation factors to talin/β3-integrin-enriched adhesion complexes in spreading and migrating mammalian cells. Biol. Cell 102, 265–276 (2010).
Willett, M., Brocard, M., Davide, A. & Morley, S. J. Translation initiation factors and active sites of protein synthesis co-localize at the leading edge of migrating fibroblasts. Biochem. J. 438, 217–227 (2011).
Mellacheruvu, D. et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat. Methods 10, 730–736 (2013).
Bachir, A. I. et al. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr. Biol. 24, 1845–1853 (2014).
Etheridge, T. et al. The integrin-adhesome is required to maintain muscle structure, mitochondrial ATP production, and movement forces in Caenorhabditis elegans. FASEB J. 29, 1235–1246 (2015).
Bulgakova, N. A., Klapholz, B. & Brown, N. H. Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr. Opin. Cell Biol. 24, 702–712 (2012).
Wickström, S. A., Lange, A., Montanez, E. & Fässler, R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29, 281–291 (2010).
Sebé-Pedrós, A., Roger, A. J., Lang, F. B., King, N. & Ruiz-Trillo, I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl Acad. Sci. USA 107, 10142–10147 (2010).
Meller, J. et al. Emergence and subsequent functional specialization of kindlins during evolution of cell adhesiveness. Mol. Biol. Cell 26, 786–796 (2015).
Kanchanawong, P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010).
Kelly, D. F. & Taylor, K. A. Identification of the β1-integrin binding site on α-actinin by cryoelectron microscopy. J. Struct. Biol. 149, 290–302 (2005).
Otey, C. A., Pavalko, F. M. & Burridge, K. An interaction between α-actinin and the β1 integrin subunit in vitro. J. Cell Biol. 111, 721–729 (1990).
Choi, C. K. et al. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 10, 1039–1050 (2008).
Rossier, O. et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 14, 1057–1067 (2012).
Zaidel-Bar, R., Cohen, M., Addadi, L. & Geiger, B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 32, 416–420 (2004).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Bass, M. D. et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 117, 527–538 (2007).
Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 (1996).
Perkins, D. N., Pappin, D. J., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Gray, K. A., Yates, B., Seal, R. L., Wright, M. W. & Bruford, E. A. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 43, D1079–D1085 (2015).
Mitchell, A. et al. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 43, D213–D221 (2015).
de Hoon, M. J. L., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Saldanha, A. J. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004).
Saeed, A. I. et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378 (2003).
Cline, M. S. et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2, 2366–2382 (2007).
Assenov, Y., Ramírez, F., Schelhorn, S.-E., Lengauer, T. & Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 24, 282–284 (2008).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Zeeberg, B. R. et al. High-Throughput GoMiner, an ‘industrial-strength’ integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of Common Variable Immune Deficiency (CVID). BMC Bioinformatics 6, 168 (2005).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Vizcaíno, J. A. et al. The Proteomics Identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 (2013).
Acknowledgements
We thank S. E. Craig for technical assistance, J. N. Selley for bioinformatic support, M. Manca for preliminary data analysis, P. March, S. Marsden and E. Zindy for assistance with microscopy, the PRIDE team for assistance with MS data deposition and G. Jacquemet, M. C. Jones and A. P. Mould for discussions. We are grateful to K. Clark, M. L. Cutler, I. J. Fidler, M. E. Hemler, D. Vestweber, J. A. Wilkins and K. M. Yamada for reagents. This work was supported by the Wellcome Trust (grant 092015 to M.J.H.), a Wellcome Trust Institutional Strategic Support Fund award (grant 097820 to the University of Manchester) and a Biotechnology and Biological Sciences Research Council studentship from the Systems Biology Doctoral Training Centre (to E.R.H.). The mass spectrometers and microscopes used in this study were purchased with grants from the Biotechnology and Biological Sciences Research Council, Wellcome Trust and the University of Manchester Strategic Fund. Data are available from ProteomeXchange with identifiers PXD000018, PXD002159 and PXD002129.
Author information
Authors and Affiliations
Contributions
A.B. and M.J.H. conceived the project; E.R.H., A.B., J.A.A., D.H.J.N., A.M.-F., J.D.H. and M.J.H. designed the experiments and interpreted the results; E.R.H., A.B., J.A.A., D.H.J.N., A.M.-F., J.R., E.J.K., N.R.P. and J.D.H. performed the experiments and analysed the data; A.B., S.W. and D.K. carried out mass spectrometry; E.R.H., A.B., J.D.H. and M.J.H. wrote the paper; all authors commented on the manuscript and approved the final version. E.R.H. and A.B. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparison of FN-enriched IAC proteomes.
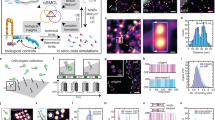
(a) Seven proteomic datasets of FN-enriched IACs were analysed by unsupervised hierarchical clustering. The binary heat map shows proteins at least two-fold enriched to FN over the negative control (red). Dataset occurrence is plotted for each protein (rainbow), and literature-curated adhesome4 components are indicated by purple bars. Details of the proteomic datasets are provided in Supplementary Table 1. (b) Dendogram illustrating the clustering of the FN-enriched IAC proteomes shown in a. Dataset dissimilarity is measured by Jaccard distance. (c) Pairwise overlaps of FN-enriched proteins identified in the seven proteomic datasets and the literature-curated adhesome were measured by Jaccard coefficient and are displayed as a hierarchically clustered heatmap (lower diagonal matrix; blue). Numbers of proteins in each overlap set are indicated (upper diagonal matrix). (d) FN-enriched proteins identified in the seven proteomic datasets were analysed by principal component analysis. A plot of the first two principal components is shown. K562, human chronic myelogenous leukaemia cells11; MEF, mouse embryonic fibroblast cells (this study); A375, human malignant melanoma cells14; HFF, human foreskin fibroblast cells13; MKF1, mouse kidney fibroblast cells15; MKF2 and MKF3, mouse kidney fibroblast cells16.
Supplementary Figure 2 Topological analysis of the meta-adhesome interaction network.
(a) Clustered protein–protein interaction network model of the meta-adhesome. The largest connected graph component is displayed, comprising 11,430 interactions (black lines; edges) between 2,035 proteins (circles; nodes). Node size is proportional to degree and node colour is proportional to betweenness centrality. Black node borders indicate literature-curated adhesome4 components, which are labelled with gene names. (b) Betweenness centrality (a measure of the control a node exerts over the interactions of other nodes in the network) for each protein is plotted according to the number of datasets in which it was identified. Box-and-whisker plot shows the median (line), mean (plus sign), 25th and 75th percentiles (box) and 5th and 95th percentiles (whiskers) (n = 1,117, 518, 238, 102, 33, 25 and 10 mapped proteins identified in 1–7 datasets, respectively, with degree ≥1). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; Kruskal–Wallis test with Dunn’s post hoc correction (see Supplementary Table 15 for statistics source data).
Supplementary Figure 3 Functional enrichment map of the meta-adhesome.
(a) Overrepresented biological process terms from proteins identified in the meta-adhesome were hierarchically clustered according to proteomic dataset occurrence. This identified clusters of similarly detected proteins associated with a similar set of functional terms. (b) The two clusters containing proteins detected in the most datasets (grey boxes in a; 1, 2) are shown in detail. Proteins are labelled with gene names for clarity (see Supplementary Table 3 for details).
Supplementary Figure 4 Comparison of IAC proteomes in the consensus adhesome.
Proteins identified in the consensus adhesome were analysed by unsupervised hierarchical clustering. The binary heat map shows proteins at least two-fold enriched to FN over the negative control (red). Dataset occurrence is plotted for each protein (rainbow), literature-curated adhesome4 components are indicated by purple bars, and the presence of a LIM domain is indicated by grey bars. Dataset dissimilarity is measured by Pearson correlation. The numbers of consensus adhesome proteins identified in each IAC proteome are displayed below the heat map. Details of the proteomic datasets are provided in Supplementary Table 1, and details of proteins identified in the consensus adhesome are provided in Supplementary Table 4. K562, human chronic myelogenous leukaemia cells11; MEF, mouse embryonic fibroblast cells (this study); A375, human malignant melanoma cells14; HFF, human foreskin fibroblast cells13; MKF1, mouse kidney fibroblast cells15; MKF2 and MKF3, mouse kidney fibroblast cells16.
Supplementary Figure 5 Hierarchical clustering analysis of meta-adhesome proteins identified during IAC assembly.
IACs were isolated from K562 cells in biological duplicate after 3, 9 and 32 min incubation with FN-coated beads and analysed by MS (data are from 2 independent experiments; see Supplementary Table 11). Throughout IAC maturation, 1,266 of the 2,412 meta-adhesome proteins were identified and were analysed by unsupervised hierarchical clustering, revealing distinct temporal profiles of protein recruitment to IACs. Quantitative heat map displays mean spectral counts as a proportion of the maximum spectral count for each given protein. Twelve clusters were chosen on the basis of a Pearson correlation threshold greater than 0.8, labelled SA1–12, and are indicated by blue and green bars. Literature-curated adhesome4 and consensus adhesome proteins identified in each cluster are indicated by gene name (italic, literature-curated adhesome; regular, consensus adhesome; bold, literature-curated adhesome and consensus adhesome). Literature-curated adhesome proteins that interact with consensus adhesome molecules in interaction network analyses are indicated by an asterisk (see Supplementary Table 7 for details). Clusters are shown alongside corresponding profile plots, with the mean temporal profile for each cluster indicated by a red line. The most significantly overrepresented functional annotations for selected clusters are listed. Full details of enriched functional terms are provided in Supplementary Table 13.
Supplementary Figure 6 Hierarchical clustering analysis of meta-adhesome proteins identified during IAC disassembly.
(a) IACs were isolated from adherent U2OS cells in biological triplicate on nocodazole removal and 5, 10 and 15 min after nocodazole washout to examine changes in IAC composition throughout IAC disruption32. Isolated IACs at each time point were analysed by MS (data are from 3 independent experiments; see Supplementary Table 12). Throughout IAC disassembly, 455 of the 2,412 meta-adhesome proteins were identified and were analysed by unsupervised hierarchical clustering, revealing distinct temporal profiles of protein dissociation from IACs. Quantitative heat map displays mean spectral counts as a proportion of the maximum spectral count for each given protein. Seventeen clusters were chosen on the basis of a Pearson correlation threshold greater than 0.8, labelled SD1–17, and are indicated by blue and green bars. Literature-curated adhesome4 and consensus adhesome proteins identified in each cluster are indicated by gene name (italic, literature-curated adhesome; regular, consensus adhesome; bold, literature-curated adhesome and consensus adhesome). Literature-curated adhesome proteins that interact with consensus adhesome molecules in interaction network analyses are indicated by an asterisk (see Supplementary Table 7 for details). Clusters are shown alongside corresponding profile plots, with the mean temporal profile for each cluster indicated by a red line. The most significantly overrepresented functional annotations for selected clusters are listed. Full details of enriched functional terms are provided in Supplementary Table 14. (b,c) Area-proportional Venn diagrams showing the overlap between the meta-adhesome and proteins identified by MS during IAC assembly (b) or IAC disassembly (c). For each set, the total number of proteins (black text) and the number of proteins identified in the consensus adhesome (bold red text) is indicated.
Supplementary Figure 7 Changes in additional consensus adhesome components during IAC disassembly.
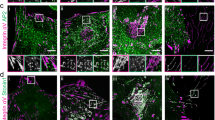
(a) To examine IAC dynamics during microtubule-induced IAC disassembly32, HFF cells treated with DMSO, 10 μM nocodazole or after nocodazole removal at different times were stained for phospho-paxillinY 118, paxillin, phospho-FAKY 397 andβ1 integrin. Representative images are shown. Scale bars, 20 μm. (b–e) Quantification of images in a. Phospho-paxillinY 118 (b), paxillin (c), phospho-FAKY 397 (d) and β1 integrin (e) levels were quantified as a proportion of total cell area. Box-and-whisker plots show median (line), mean (plus sign), 25th and 75th percentiles (box) and 5th and 95th percentiles (whiskers) (n = 10 cells per condition from one independent experiment). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001; Kruskal–Wallis test with Dunn’s post hoc correction (see Supplementary Table 15 for statistics source data). Noc, nocodazole.
Supplementary information
Supplementary Information
Supplementary Information (PDF 7646 kb)
Supplementary Table 1
Supplementary Information (XLSX 14 kb)
Supplementary Table 2
Supplementary Information (XLSX 304 kb)
Supplementary Table 3
Supplementary Information (XLSX 329 kb)
Supplementary Table 4
Supplementary Information (XLSX 28 kb)
Supplementary Table 5
Supplementary Information (XLSX 14 kb)
Supplementary Table 6
Supplementary Information (XLSX 18 kb)
Supplementary Table 7
Supplementary Information (XLSX 13 kb)
Supplementary Table 8
Supplementary Information (XLSX 376 kb)
Supplementary Table 9
Supplementary Information (XLSX 38 kb)
Supplementary Table 10
Supplementary Information (XLSX 23 kb)
Supplementary Table 11
Supplementary Information (XLSX 382 kb)
Supplementary Table 12
Supplementary Information (XLSX 133 kb)
Supplementary Table 13
Supplementary Information (XLSX 47 kb)
Supplementary Table 14
Supplementary Information (XLSX 28 kb)
Supplementary Table 15
Supplementary Information (XLSX 319 kb)
Rights and permissions
About this article
Cite this article
Horton, E., Byron, A., Askari, J. et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 17, 1577–1587 (2015). https://doi.org/10.1038/ncb3257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3257
This article is cited by
-
CD248 promotes migration and metastasis of osteosarcoma through ITGB1-mediated FAK-paxillin pathway activation
BMC Cancer (2023)
-
Talin2 and KANK2 functionally interact to regulate microtubule dynamics, paclitaxel sensitivity and cell migration in the MDA-MB-435S melanoma cell line
Cellular & Molecular Biology Letters (2023)
-
Extracellular matrix-induced signaling pathways in mesenchymal stem/stromal cells
Cell Communication and Signaling (2023)
-
Integrins and their potential roles in mammalian pregnancy
Journal of Animal Science and Biotechnology (2023)
-
Organization, dynamics and mechanoregulation of integrin-mediated cell–ECM adhesions
Nature Reviews Molecular Cell Biology (2023)