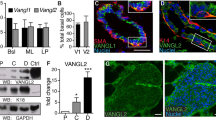
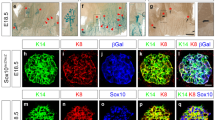
Abstract
The Par polarity proteins play key roles in asymmetric division of Drosophila melanogaster stem cells; however, whether the same mechanisms control stem cells in mammals is controversial. Although necessary for mammary gland morphogenesis, Par3 is not essential for mammary stem cell function. We discovered that, instead, a previously uncharacterized protein, Par3-like (Par3L), is vital for mammary gland stem cell maintenance. Par3L function has been mysterious because, unlike Par3, it does not interact with atypical protein kinase C or the Par6 polarity protein. We found that Par3L is expressed by multipotent stem cells in the terminal end buds of murine mammary glands. Ablation of Par3L resulted in rapid and profound stem cell loss. Unexpectedly, Par3L, but not Par3, binds to the tumour suppressor protein Lkb1 and inhibits its kinase activity. This interaction is key for the function of Par3L in mammary stem cell maintenance. Our data reveal insights into a link between cell polarity proteins and stem cell survival, and uncover a biological function for Par3L.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
30 May 2014
In the version of this Article originally published, the scale bars in Fig. 2c should have been labelled '3 mm'. In addition, a small red arrow indicating the location of the mutation was missing from Fig. 5c (shown below). These errors have now been corrected in the online versions of the Article.
References
Knoblich, J. A. Mechanisms of asymmetric stem cell division. Cell 132, 583–597 (2008).
Goldstein, B. & Macara, I. G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609–622 (2007).
Bultje, R. S. et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 63, 189–202 (2009).
McCaffrey, L. M. & Macara, I. G. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 23, 1450–1460 (2009).
Sengupta, A., Duran, A., Ishikawa, E., Florian, M. C. & Dunn, S. K. et al. Atypical protein kinase C (aPKCζ and aPKCλ) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc. Natl Acad. Sci. USA 108, 9957–9962 (2011).
Munson, C. et al. Regulation of neurocoel morphogenesis by Pard6 γ b. Dev. Biol. 324, 41–54 (2008).
Gao, L., Macara, I. G. & Joberty, G. Multiple splice variants of Par3 and of a novel related gene, Par3L, produce proteins with different binding properties. Gene 294, 99–107 (2002).
Kohjima, M., Noda, Y., Takeya, R., Saito, N., Takeuchi, K. & Sumimoto, H. PAR3β, a novel homologue of the cell polarity protein PAR3, localizes to tight junctions. Biochem. Biophys. Res. Commun. 299, 641–646 (2002).
Rohrschneider, L. R., Custodio, J. M., Anderson, T. A., Miller, C. P. & Gu, H. The intron 5/6 promoter region of the ship1 gene regulates expression in stem/progenitor cells of the mouse embryo. Dev. Biol. 283, 503–521 (2005).
Bai, L. & Rohrschneider, L. R. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev. 24, 1882–1892 (2010).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 (2006).
Cheng, H. et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci. Signal 2, ra35 (2009).
Karuman, P. et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 7, 1307–1319 (2001).
Denning, D. P., Hatch, V. & Horvitz, H. R. Programmed elimination of cells by caspase-independent cell extrusion in C elegans. Nature 488, 226–230 (2012).
Nakada, D., Saunders, T. L. & Morrison, S. J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653–658 (2010).
Gurumurthy, S., Xie, S. Z., Alagesan, B., Kim, J. & Yusuf, R. Z. et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659–663 (2010).
Gan, B. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704 (2010).
Partanen, J. I. et al. Tumor suppressor function of Liver kinase B1 (Lkb1) is linked to regulation of epithelial integrity. Proc. Natl Acad. Sci. USA 109, 388–397 (2012).
Brill, B., Boecher, N., Groner, B. & Shemanko, C. S. A sparing procedure to clear the mouse mammary fat pad of epithelial components for transplantation analysis. Lab. Anim. 42, 104–110 (2008).
Wiznerowicz, M. & Trono, D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77, 8957–8961 (2003).
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Hu, Y. & Smyth, G. K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009).
Acknowledgements
This study was supported by NIH grant GM070902 (I.G.M.) and DoD postdoctoral fellowship W81XWH-11-1-0083 (Y.H.).
Author information
Authors and Affiliations
Contributions
Y.H. carried out the experiments; Y.H. and I.G.M. analysed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1
(a,b) Specificity of the Par3L antibody. Par3L antibody recognizes over-expressed human YFP-tagged Par3L in HEK293T cells and endogenous murine Par3L in mouse kidney lysates. (c) Par3L staining co-localizes with GFP staining in the mammary glands of 6-week old s-Ship-GFP transgenic mice. (d,e) Immunoblot to test the efficiency of shPar3L hairpin RNAs. HEK293T cells over-expressing mouse Par3L gene were infected by Par3L hairpin virus. Par3L shRNA 1 reduced Par3L expression by 70%, and shPar3L#2 completely depleted Par3L protein. Graph represents mean ± s.e.m. for n = 3 independent experiments. All full scan images of western blots can be found in Supplementary Fig. 6.
Supplementary Figure 2
(a) Scheme of serial colony-forming assay. Mammary gland cells were isolated from adult female C3H or s-SHIP-GFP transgenic mice. Cells were cultured for two days in suspension after virus infection to enable recovery. Before Matrigel culture cells were dissociated in fresh 0.05% trypsin for 45 min at 37 °C to obtained single cells. Three hundred to 1,000 cells in 5 μl of medium were mixed with 45 μl growth factor reduced phenol-red free Matrigel. The mixture were laid on the bottom of ultralow attachment 24-well cluster (Corning) as a drop and solidified at 37 °C for 30 min. The solidified Matrigel drops were covered by 500 μl of mammosphere medium. The cultures were fed every other day. Colonies were scored and imaged using an Olympus SZX16 microscope. For passage, colonies were recovered in 0.5 ml Cell Recovery Solution (BD) at 4 °C for 30 min. Single cells were obtained by trypsinization and plated in Matrigel culture as described previously. After the third passage, colonies were analyzed by immuno-staining in vitro or by transplantation. (b) Summary of limiting dilution assay using control tertiary colonies and a representative image of a GFP+ mammary gland regenerated in the limiting dilution assay from these control cells. Mammary gland cells expressing shLuc were used for the serial colony-forming assay. After the third passage, colonies of the indicated numbers were injected into cleared fat pad for the limiting dilution assay. Mammary gland regeneration was tested in 8 cleared mammary fat pads. (c) Scheme of competition assay. Mammary gland cells were isolated from adult female C3H mice. The cells were infected with mApple-ShLuc virus, EGFP-ShLuc virus or EGFP-ShPar3L#2 virus. After 2 days recovery in suspension culture, 50,000 mApple-ShLuc virus infected cells were combined with an equal number of EGFP-ShLuc virus infected cells or EGFP-ShPar3L#2 virus infected cells and injected into cleared fat pads of 3 week old C3H mice. Mammary glands were scored for outgrowth after 6 weeks.
Supplementary Figure 3
Representative images of mature mammary ducts stained for CK8, CK14, or cleaved Caspase-3. Those mammary ducts that grew out from cells depleted of Par3L show a normal morphology with a single layer of luminal epithelial cells surrounded by a single layer of myoepithelial cells. The number of cleaved Caspase-3+ cells is very low and not significantly different from the ShLuc control.
Supplementary Figure 4
(a) Scheme of mammary stem cell colony-forming assay. Mammary gland cells were isolated from 6-week old female s-SHIP-GFP transgenic mice. GFP+ mammary gland stem cells were purified by FACS sorting as described in Methods. The sorted cells were then infected with mApple-ShLuc virus or mApple-ShPar3L#2 virus. After recovery for 2 days in suspension culture the cells were plated in Matrigel. (b) Scheme of FACS for the GFP+ mammary gland stem cells. Mammary gland cells from s-SHIP-GFP transgenic mice were stained by the following lineage antibodies: PE-CD31 (MEC13.3, BD), PE-CD45 (30-F11, BD) and PE-Ter119 (TER-119, BD). 7AAD (BD) was added before sorting. GFP+PE-7AAD- single cells were collected for the experiments.
Supplementary Figure 5
The region between PDZ2 and PDZ3 on Par3L protein is necessary for the interaction between Par3L and LKB1. (a) schematic view of the Par3L truncation mutants used for co-immunoprecipitation with LKB1. (b) co-precipitation of LKB1 and Par3L truncation mutants group #1. LKB1 binding to Par3L protein required the region from PDZ2 to PDZ3 domain. (c) co-precipitation of LKB1 and Par3L truncation mutants group #2. LKB1 was unable to bind to any one of the 3 isolated PDZ domains. (d) co-precipitation of LKB1 and Par3L truncation mutants group #3. LKB1 binding to Par3L protein requires PDZ domains 2 and 3 plus the intervening region. All experiments were conducted 3× independently. All full scan images of western blots can be found in Supplementary Fig. 6.
Supplementary Figure 6
Original data for the immunoblots presented in the other figures.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1154 kb)
Supplementary Information
Supplementary Information (XLS 27 kb)
Rights and permissions
About this article
Cite this article
Huo, Y., Macara, I. The Par3-like polarity protein Par3L is essential for mammary stem cell maintenance. Nat Cell Biol 16, 526–534 (2014). https://doi.org/10.1038/ncb2969
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2969
This article is cited by
-
The Terminal End Bud: the Little Engine that Could
Journal of Mammary Gland Biology and Neoplasia (2017)
-
Murine mammary stem/progenitor cell isolation: Different method matters?
SpringerPlus (2016)
-
Apical–Basal Polarity as a Sensor for Epithelial Homeostasis: A Matter of Life and Death
Current Pathobiology Reports (2016)
-
Erratum: The Par3-like polarity protein Par3L is essential for mammary stem cell maintenance
Nature Cell Biology (2014)