Abstract
Rab GTPases play key roles in the delivery, docking and fusion of intracellular vesicles. However, the mechanism by which spatial and temporal regulation of Rab GTPase activity is controlled is poorly understood. Here we describe a mechanism by which localized calcium release through a vesicular ion channel controls Rab GTPase activity. We show that activation of P2XA, an intracellular ion channel localized to the Dictyostelium discoideum contractile vacuole system, results in calcium efflux required for downregulation of Rab11a activity and efficient vacuole fusion. Vacuole fusion and Rab11a downregulation require the activity of CnrF, an EF-hand-containing Rab GAP found in a complex with Rab11a and P2XA. CnrF Rab GAP activity for Rab11a is enhanced by the presence of calcium and the EF-hand domain. These findings suggest that P2XA activation results in vacuolar calcium release, which triggers activation of CnrF Rab GAP activity and subsequent downregulation of Rab11a to allow vacuole fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hutagalung, A. H. & Novick, P. J. Role of Rab GTPases in membrane traffic and cell physiology. Physol. Rev. 91, 119–149 (2011).
Lippe, R., Miaczynska, M., Rybin, V., Runge, A. & Zerial, M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol. Biol. Cell 12, 2219–2228 (2001).
Mizuno-Yamasaki, E., Medkova, M., Coleman, J. & Novick, P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev. Cell 18, 828–840 (2010).
Ortiz, D., Medkova, M., Walch-Solimena, C. & Novick, P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157, 1005–1015 (2002).
Pereira-Leal, J. B. & Seabra, M. C. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901 (2001).
Mizuno-Yamasaki, E., Rivera-Molina, F. & Novick, P. GTPase networks in membrane traffic. Annu. Rev. Biochem. 81, 637–659 (2012).
Hay, J. C. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep. 8, 236–240 (2007).
Adler, E. M., Augustine, G. J., Duffy, S. N. & Charlton, M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 11, 1496–1507 (1991).
Beckers, C. J. & Balch, W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J. Cell Biol. 108, 1245–1256 (1989).
Burgoyne, R. D. & Clague, M. J. Calcium and calmodulin in membrane fusion. Biochim. Biophys. Acta 1641, 137–143 (2003).
Colombo, M. I., Beron, W. & Stahl, P. D. Calmodulin regulates endosome fusion. J. Biol. Chem. 272, 7707–7712 (1997).
Holroyd, C., Kistner, U., Annaert, W. & Jahn, R. Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell 10, 3035–3044 (1999).
Peters, C. & Mayer, A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575–580 (1998).
Porat, A. & Elazar, Z. Regulation of intra-Golgi membrane transport by calcium. J. Biol. Chem. 275, 29233–29237 (2000).
Pryor, P. R., Mullock, B. M., Bright, N. A., Gray, S. R. & Luzio, J. P. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149, 1053–1062 (2000).
Chen, J. L., Ahluwalia, J. P. & Stamnes, M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 277, 35682–35687 (2002).
Flanagan, J. J. & Barlowe, C. Cysteine-disulphide cross-linking to monitor SNARE complex assembly during endoplasmic reticulum-Golgi transport. J. Biol. Chem. 281, 2281–2288 (2006).
Starai, V. J., Thorngren, N., Fratti, R. A. & Wickner, W. Ion regulation of homotypic vacuole fusion in Saccharomyces cerevisiae. J. Biol. Chem. 280, 16754–16762 (2005).
Xu, D. & Hay, J. C. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J. Cell Biol. 167, 997–1003 (2004).
Becker, M., Matzner, M. & Gerisch, G. Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. EMBO J. 18, 3305–3316 (1999).
Bush, J., Temesvari, L., Rodriguez-Paris, J., Buczynski, G. & Cardelli, J. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol. Biol. Cell 7, 1623–1638 (1996).
Du, F. et al. Regulation of contractile vacuole formation and activity in Dictyostelium. EMBO J. 27, 2064–2076 (2008).
Essid, M., Gopaldass, N., Yoshida, K., Merrifield, C. & Soldati, T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol. Biol. Cell 23, 1267–1282 (2012).
Gerald, N. J., Siano, M. & De Lozanne, A. The Dictyostelium LvsA protein is localized on the contractile vacuole and is required for osmoregulation. Traffic 3, 50–60 (2002).
Harris, E., Yoshida, K., Cardelli, J. & Bush, J. Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in Dictyostelium. J. Cell Sci. 114, 3035–3045 (2001).
Heuser, J., Zhu, Q. & Clarke, M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121, 1311–1327 (1993).
Wu, W. I., Yajnik, J., Siano, M. & De Lozanne, A. Structure-function analysis of the BEACH protein LvsA. Traffic 5, 346–355 (2004).
Moreno, S. N. & Docampo, R. The role of acidocalcisomes in parasitic protists. J. Eukaryot. Microbiol. 56, 208–213 (2009).
Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Gerisch, G., Heuser, J. & Clarke, M. Tubular-vesicular transformation in the contractile vacuole system of Dictyostelium. Cell Biol. Int. 26, 845–852 (2002).
Fountain, S. J. et al. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature 448, 200–203 (2007).
Khakh, B. S. & North, R. A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76, 51–69 (2012).
Surprenant, A. & North, R. A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 (2009).
Baines, A. et al. Functional properties of five Dictyostelium discoideum P2X receptors. J. Biol. Chem. 288, 20992–21000 (2013).
Sivaramakrishnan, V. & Fountain, S. J. A mechanism of intracellular P2X receptor activation. J. Biol. Chem. 287, 28315–28326 (2012).
Richler, E., Chaumont, S., Shigetomi, E., Sagasti, A. & Khakh, B. . Tracking transmitter-gated P2X cation channel activation in vitro and in vivo. Nat. Methods 5, 87–93 (2008).
Pan, X., Eathiraj, S., Munson, M. & Lambright, D. G. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442, 303–306 (2006).
Starovasnik, M. A., Su, D. R., Beckingham, K. & Klevit, R. E. A series of point mutations reveal interactions between the calcium-binding sites of calmodulin. Protein Sci. a Publ. Protein Soc. 1, 245–253 (1992).
Miklavc, P. et al. Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl Acad. Sci. USA 108, 14503–14508 (2011).
Qureshi, O. S., Paramasivam, A., Yu, J. C. & Murrell-Lagnado, R. D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 120, 3838–3849 (2007).
Ludlow, M. J., Durai, L. & Ennion, S. J. Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J. Biol. Chem. 284, 35227–35239 (2009).
Temesvari, L. A., Rodriguez-Paris, J. M., Bush, J. M., Zhang, L. & Cardelli, J. A. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. J. Cell Sci. 109 (Pt 6), 1479–1495 (1996).
Allen, R. D. The contractile vacuole and its membrane dynamics. Bioessays 22, 1035–1042 (2000).
Allan, C. Y. & Fisher, P. R. In vivo measurements of cytosolic calcium in Dictyostelium discoideum. Methods Mol. Biol. 571, 291–308 (2009).
Guo, W., Roth, D., Walch-Solimena, C. & Novick, P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 (1999).
Medkova, M., France, Y. E., Coleman, J. & Novick, P. The rab exchangefactor Sec2p reversibly associates with the exocyst. Mol. Biol. Cell 17, 2757–2769 (2006).
Chesneau, L. et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr. Biol. 22, 147–153 (2012).
Sussman, M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28, 9–29 (1987).
Sasaki, A. T., Chun, C., Takeda, K. & Firtel, R. A. Localized Ras signalling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 167, 505–518 (2004).
Acknowledgements
This work was supported by a Medical Research Council grant (G0900069) to R.A.N. and C.R.L.T. and a Wellcome Trust Investigator Award (WT095643) to C.R.L.T.
Author information
Authors and Affiliations
Contributions
K.P. performed most of the experiments. A.E.B. performed the electrophysiology, P2XA point mutant and GCaMP2 studies; T.K. created the CnrF Rab GAP knockout strain; N.G. performed the bioinformatic analysis of EF-hand-containing Rab GAPs; L.B. created the P2XA point mutants for expression in HEK cells. C.R.L.T. and R.A.N. conceived of the study and wrote the manuscript. All authors discussed the results, and contributed to writing or commenting on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 A P2XA deletion mutant exhibits defects in osmoregulation.
A. Schematic to show the generation of a P2XA deletion construct for use in homologous recombination. 1.6kb downstream and 1.4kb upstream of the P2XA coding sequence were amplified and cloned either side of the floxed blasticidin cassette in pLPBLP. B. Bright field images of cells in KK2, and after 10min and 60min after changing the media from KK2 to water to induce osmotic shock. Wild-type cells round up within 10 minutes, but fully recover to their normal shape within 60 minutes in water. P2XA− cells also round up within 10 minutes, but fail to recover their shape even after 60 minutes in water. P2XA− cells expressing P2XA:GFP behave like wild-type cells. Wild-type cells treated with 10uM copper fail to recover their shape after 60 minutes in water. Scale bar = 20 μm C. Time course of cell rounding and recovery. Wild-type cells (black squares, solid line) round up for 20-30 min and then regain their normal shape by 40 minutes. P2XA− cells (red square, solid line) become rounder than wild type cells and stay round throughout the entire time course. P2XA− cells expressing P2XA:GFP (red square, dashed line) are similar to wild-type cells, but get slightly rounder and take longer to regain their shape. Wild-type cells treated with 10 μM copper (black square, dashed line) stay round throughout the entire time course. Error bars represent s.e.m. and results are means of n = 3 independent experiments, each with 120 cells. Statistical source data for Supplementary Fig 1C can be found in Supplementary Table 2.
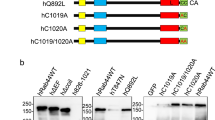
Supplementary Figure 2 P2XA mutants correctly localise to the contractile vacuole.
Fluorescent images of RFP tagged mutants expressed in wild-type Dictyostelium. Fluorescence shows that P2XA(R63A), P2XA(R285K) and P2XA(K67A/K289A) all correctly localise to the contractile vacuole in Dictyostelium. Scale bar = 5 μm.
Supplementary Figure 3 P2XA is specifically expressed on CV’s at all stages of the cycle and undergoes a ‘ring to patch’ transition
A. Localization of Rab7:GFP and P2XA:RFP in wild-type cells. No co-localization is observed. Scale bar = 5 μm B. Localization of Dajumin:GFP and P2XA:RFP in wild-type cells at the maturation (top panel) and ‘ring to patch’ (bottom panel) stages of the CV cycle. P2XA:RFP co-localizes with Dajumin:GFP during maturation, but localization of P2XA:RFP is restricted to the plasma membrane contact site of the CV during the ring to patch transition stage. Scale bar = 5 μm C. Localization of drainin:GFP and P2XA:RFP in wild-type cells at the maturation (top panel) and ‘ring to patch’ (bottom panel) stages of the CV cycle. P2XA:RFP co-localizes with drainin:GFP at all stages of the cycle. Scale bar = 5 μm D. Localization of rab8a:GFP and P2XA:RFP in wild-type cells. P2XA:RFP co-localizes with rab8a:GFP during the ‘ring to patch’ transition stage of the CV cycle. Scale bar = 5 μm.
Supplementary Figure 4 GCaMP2 acts as a calcium sensor in HEK cells
A. Confocal images of P2XA-GCaMP2 expressed in HEK cells before (control), during (+ATP) and after (wash) stimulation with ATP (1 mM-10 μM). Increases in fluorescence can be seen when stimulated with 100 μM, 300 μM and 1mM ATP. B. GCaMP2 acts as a sensor for P2XA activity. Fluorescent confocal images of HEK cells expressing P2XA-GCaMP2, P2XA-GFP and P2XA(K67A/K289A)-GCaMP2 over time. Following stimulation with 1mM ATP, P2XA-GCaMP2 cells show a transient increase in fluorescence at 20 seconds, whilst there is no change in fluorescence for P2XA-GFP or P2XA(K67A/K289A)-GCaMP2. Scale bar = 10 μm.
Supplementary Figure 5 GCaMP2 acts as a calcium sensor Dictyostelium cells
A. Time course of cell rounding and recovery of P2XA− cells expressing P2XA-GCaMP2. Over-expression of P2X-GCaMP2 in the P2XA− background (green) rescues the osmoregulation phenotype so that cells transiently round up after 10 mins in water but are able to recover their shape within 60 mins. Error bars represent s.e.m. and results are means of n = 4 independent experiments. Statistical source data for Supplementary Fig 5A can be found in Supplementary Table 2. B. Fluorescence LUT images of Dictyostelium cells expressing Actin15-GCaMP2 or Actin15-GFP. Increases in fluorescence are observed in cells expressing Actin15-GCAMP2 after ATP stimulation (30 μM) in the presence of extracellular Ca 2+ only. No changes in fluorescence are observed in cells expressing Actin15-GFP in the presence or absence of Ca 2+. Scale bar = 15 μm C. Plot of changes in fluorescence of cells expressing Actin15-GCaMP2 or Actin15-GFP over time following 30 μM ATP stimulation. Error bars represent s.e.m. and results are means of n = 5 independent experiments, each with 5 cells. Increases in fluorescence are observed in cells expressing Actin15-GCaMP2 only in the presence of extracellular Ca 2+.
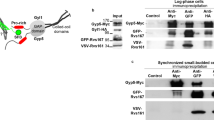
Supplementary Figure 6 Validation of Rab11a–GTP specific antibody
A. The anti rab11-GTP antibody is specific for rab11-GTP in vitro. Rab11a-GST or Rab8-GST were loaded with either GTPγS or GDP, incubated with anti rab11-GTP antibody and then immunoprecipitated using protein A/G agarose beads. Bound protein was probed with an anti-GST antibody. The anti rab11-GTP antibody could only immunoprecipitate bacterially expressed rab11a when loaded with GTP. B. The anti rab11-GTP antibody is specific for rab11a-GTP in vivo. Immunoprecipitation of rab11a-GTP from wild-type cells expressing Rab11a-RFP, Rab7-RFP or Rab8-RFP. Lysate from each strain was incubated with anti rab11-GTP antibody and immunoprecipitated using protein A/G agarose beads. Bound and unbound proteins were probed with an anti RFP antibody. (I = input, B = bound, NB = not bound). The anti rab11-GTP antibody could only immunoprecipitate RFP from cells expressing rab11a-RFP. C. The anti rab11-GTP antibody works in the linear range for protocols used throughout the manuscript. Top panel: Lysate from AX4 cells expressing rab11a-RFP was used neat, at a 1:2 dilution or a 1:4 dilution for immunoprecipitation of rab11a-GTP using the anti rab11-GTP antibody. Bottom panel: Densitometry analyses of bound (rab11a-GTP) and unbound (rab11a-GDP) bands reveal that the GTP/GDP ratios remain constant for each sample dilution. Error bars represent s.e.m. and results are means of n = 3 independent experiments. Statistical source data for Supplementary Fig 6C can be found in Supplementary Table 2.
Supplementary Figure 7 An evolutionarily conserved group of Rab GAPs containing a TBC Rab GAP domain and EF hands.
A conserved group of Rab GAPs containing a TBC Rab GAP domain and EF hands from different phyla. Proteins from D. discoidium (CnrF, DDB_G0295717 and DDB_G0275421), S. pombe (NP_596678), P. tetraurelia (XP_001441542), D. rerio (NP_001120987), X. tropicalis (NP_001107313), M. musculus (NP_001104774), H. sapiens (NP_942568) are shown. A. Protein structure is conserved throughout evolution. B. Alignment of the TBC Rab GAP domain. Homologous sequences were identified using the conserved domains database (Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, Deweese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011 Jan;39(Database issue):D225-9. Epub 2010 Nov 24. [PubMed PMID: 21109532]). The alignment was computed using MUSCLE requesting 16 iterations (MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004, 32:1792–1797.). The aligned TBC Rab GAP domain was extracted using CLC Genomics workbench (www.CLCbio.com). C. Alignment of the EF hand domain. Homologous sequences were identified using the conserved domains database (CDD ref). The alignment was computed using MUSCLE requesting 16 iterations (MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004, 32:1792–1797.). The aligned EF hand domain was extracted using CLC Genomics workbench (www.CLCbio.com).
Supplementary information
Supplementary Information
Supplementary Information (PDF 1951 kb)
Supplementary Table 1
Supplementary Information (XLSX 9 kb)
Supplementary Table 2
Supplementary Information (XLSX 81 kb)
dajumin: GFP expression in wild type cells
Live cell fluorescence imaging of a wild-type cell expressing dajumin:GFP during CV cycling (imaged between 0 and 330 seconds after osmotic shock). (MOV 521 kb)
dajumin: GFP expression in P2XA− cells
Live cell fluorescence imaging of a P2XA− cell expressing dajumin:GFP during CV cycling (imaged between 60 and 210 seconds after osmotic shock). (AVI 28780 kb)
drainin: GFP expression in wild type cells
Live cell fluorescence imaging of a wild-type cell expressing drainin:GFP during CV cycling (imaged between 60 and 190 seconds after osmotic shock). (MOV 468 kb)
drainin: GFP expression in P2XA− cells
Live cell fluorescence imaging of a P2XA− cell expressing drainin:GFP during CV cycling (imaged between 60 and 190 seconds after osmotic shock). (MOV 426 kb)
rab8: GFP expression in wild type cells
Live cell fluorescence imaging of a wild-type cell expressing rab8:GFP during CV cycling (imaged between 240 and 350 seconds after osmotic shock). (MOV 546 kb)
rab8: GFP expression in P2XA− cells
Live cell fluorescence imaging of a P2XA− cell expressing rab8:GFP during CV cycling (imaged between 240 and 350 seconds after osmotic shock). (MOV 604 kb)
P2XA-GCaMP2 in HEK cells
Live cell fluorescence imaging of a HEK cells expressing P2XA-GCaMP2 and stimulated with ATP (1mM) which causes a transient increase in fluorescence. (AVI 547 kb)
P2XA-GFP in HEK cells
Live cell fluorescence imaging of a HEK cells expressing P2XA-GFP and stimulated with ATP (1mM). No increase in fluorescence is observed. (AVI 410 kb)
P2XA-GCaMP2/P2XA-RFP co-expressing cell
Live cell fluorescence imaging of a wild-type cells co-expressing P2XA-GCaMP2 and P2XA-RFP and imaged in water during osmotic shock. (AVI 27057 kb)
P2XA-GFP/P2XA-RFP co-expressing cell
Live cell fluorescence imaging of a wild-type cells co-expressing P2XA-GFP and P2XA-RFP and imaged in water during osmotic shock. (AVI 1126 kb)
P2XA(K67A/K289A)-GCaMP2/P2XA(K67A/K289A)-RFP co-expressing cell
Live cell fluorescence imaging of a wild-type cells co-expressing mutant (K67A/K289A) P2XA-GCaMP2 and mutant (K67A/K289A) P2XA-RFP and imaged in water during osmotic shock. (AVI 16510 kb)
Rights and permissions
About this article
Cite this article
Parkinson, K., Baines, A., Keller, T. et al. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat Cell Biol 16, 87–98 (2014). https://doi.org/10.1038/ncb2887
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2887
This article is cited by
-
Ectopic ATP synthase stimulates the secretion of extracellular vesicles in cancer cells
Communications Biology (2023)
-
A two-pore channel protein required for regulating mTORC1 activity on starvation
BMC Biology (2020)
-
Drosophila taste neurons as an agonist-screening platform for P2X receptors
Scientific Reports (2020)
-
5-methylcytosine promotes mRNA export — NSUN2 as the methyltransferase and ALYREF as an m5C reader
Cell Research (2017)
-
Dictyostelium discoideum RabS and Rab2 colocalize with the Golgi and contractile vacuole system and regulate osmoregulation
Journal of Biosciences (2016)