Abstract
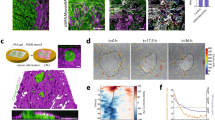
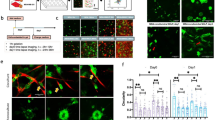
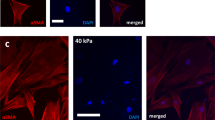
To learn more about cancer-associated fibroblasts (CAFs), we have isolated fibroblasts from different stages of breast cancer progression and analysed their function and gene expression. These analyses reveal that activation of the YAP transcription factor is a signature feature of CAFs. YAP function is required for CAFs to promote matrix stiffening, cancer cell invasion and angiogenesis. Remodelling of the ECM and promotion of cancer cell invasion requires the actomyosin cytoskeleton. YAP regulates the expression of several cytoskeletal regulators, including ANLN and DIAPH3, and controls the protein levels of MYL9 (also known as MLC2). Matrix stiffening further enhances YAP activation, thus establishing a feed-forward self-reinforcing loop that helps to maintain the CAF phenotype. Actomyosin contractility and Src function are required for YAP activation by stiff matrices. Further, transient ROCK inhibition is able to disrupt the feed-forward loop, leading to a long-lasting reversion of the CAF phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Bhowmick, N. A. & Moses, H. L. Tumour-stroma interactions. Curr. Opin. Gen. Dev. 15, 97–101 (2005).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Calvo, F. & Sahai, E. Cell communication networks in cancer invasion. Curr. Opin. Cell Biol. 23, 621–629 (2011).
Finak, G. et al. Gene expression signatures of morphologically normal breast tissue identify basal-like tumours. Breast Cancer Res. 8, R58 (2006).
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (2007).
Levental, K. R. et al. Matrix crosslinking forces tumour progression by enhancing integrin signalling. Cell 139, 891–906 (2009).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Guilluy, C. et al. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 722–727 (2011).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Lamar, J. M. et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl Acad. Sci. USA 109, E2441–E2450 (2012).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Sugimoto, H., Mundel, T. M., Kieran, M. W. & Kalluri, R. Identification of fibroblast heterogeneity in the tumour microenvironment. Cancer Biol. Therapy 5, 1640–1646 (2006).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumours by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12, 954–961 (1992).
Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461, 1084–1091 (2009).
Kim, J. W. et al. Loss of fibroblast HIF-1alpha accelerates tumourigenesis. Cancer Res. 72, 3187–3195 (2012).
Vousden, K. H. HPV E6: ensuring all’s well at the end. Trends Microbiol. 4, 337–338 (1996).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Shree, T. et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 (2011).
Subramanian, A., Kuehn, H., Gould, J., Tamayo, P. & Mesirov, J. P. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 23, 3251–3253 (2007).
Roepman, P. et al. Dissection of a metastatic gene expression signature into distinct components. Genome Biol. 7, R117 (2006).
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 (2008).
Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumour-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Chaudhry, S. I. et al. Autocrine IL-1beta-TRAF6 signalling promotes squamous cell carcinoma invasion through paracrine TNFalpha signalling to carcinoma-associated fibroblasts. Oncogene 32, 747–758 (2013).
Kojima, Y. et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signalling drives the evolution of tumour-promoting mammary stromal myofibroblasts. Proc. Natl Acad. Sci. USA 107, 20009–20014 (2010).
Zhang, H. et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J. Biol. Chem. 284, 13355–13362 (2009).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes. Dev. 22, 1962–1971 (2008).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Descot, A. et al. Negative regulation of the EGFR-MAPK cascade by actin-MAL-mediated Mig6/Errfi-1 induction. Mol. Cell 35, 291–304 (2009).
Selvaraj, A. & Prywes, R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol. Biol. 5, 13 (2004).
McGee, K. M., Vartiainen, M. K., Khaw, P. T., Treisman, R. & Bailly, M. Nuclear transport of the serum response factor coactivator MRTF-A is downregulated at tensional homeostasis. EMBO Rep. 12, 963–970 (2011).
Pan, D. The hippo signalling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Zhao, B., Tumaneng, K. & Guan, K. L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 (2011).
Basu, S., Totty, N. F., Irwin, M. S., Sudol, M. & Downward, J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 (2003).
Fernandez, B. G. et al. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138, 2337–2346 (2011).
Densham, R. M. et al. MST kinases monitor actin cytoskeletal integrity and signal via c-Jun N-terminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol. Cell. Biol. 29, 6380–6390 (2009).
Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 (2011).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011).
Levy, D., Adamovich, Y., Reuven, N. & Shaul, Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell 29, 350–361 (2008).
Azab, A. K. et al. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood 114, 619–629 (2009).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signalling. Cell 150, 780–791 (2012).
Tamm, C., Bower, N. & Anneren, C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signalling pathway downstream of LIF. J. Cell Sci. 124, 1136–1144 (2011).
Carragher, N. O. & Frame, M. C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends in Cell Biol. 14, 241–249 (2004).
Matallanas, D. et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumour suppressor protein. Mol. Cell 27, 962–975 (2007).
Harris, A. R. & Charras, G. T. Experimental validation of atomic force microscopy-based cell elasticity measurements. Nanotechnology 22, 345102 (2011).
Bild, A. H. et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439, 353–357 (2006).
Buess, M. et al. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 8, R191 (2007).
Rajski, M. et al. IGF-I induced genes in stromal fibroblasts predict the clinical outcome of breast and lung cancer patients. BMC Med. 8, 1 (2010).
Mazzone, M. et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc. Natl Acad. Sci. USA 107, 5012–5017 (2010).
Klapholz-Brown, Z., Walmsley, G. G., Nusse, Y. M., Nusse, R. & Brown, P. O. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PloS One 2, e945 (2007).
Kaposi-Novak, P. et al. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J. Clin. Invest. 116, 1582–1595 (2006).
Park, B. K. et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat. Med. 13, 62–69 (2007).
Farmer, P. et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 15, 68–74 (2009).
Acknowledgements
F.C., N.E., S.H., R.P.J., S.I.C., K.H. and E.S. are financially supported by Cancer Research UK. A.G-G. was financially supported by a Royal Society Newton Fellowship, E.M. is in receipt of a Dorothy Hodgkins Postgraduate Award (DHPA) from the Engineering and Physical Sciences Research Council. G.C. is in receipt of a Royal Society University Research Fellowship. We thank N. Tapon, B. Thompson and laboratory members for help and advice throughout this work.
Author information
Authors and Affiliations
Contributions
F.C. carried out all the experiments except those noted otherwise. N.E. performed all the quantitative real-time PCR analyses and generated data for Fig. 7e. E.S. generated data for Figs 3, 6g and 7b. E.M. and G.C. performed all the AFM analyses. A.G-G. and S.H. isolated and immortalized several breast and human CAFs. R.P.J. wrote the script for organotypic invasion quantification and helped analyse data for Supplementary Fig. S2c,e. S.I.C., K.H. and P.W. provided clinical material. F.C. and E.S. conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1463 kb)
Supplementary Table 1
Supplementary Information (XLSX 10 kb)
Supplementary Table 2
Supplementary Information (XLSX 31 kb)
Supplementary Table 3
Supplementary Information (XLSX 11 kb)
Supplementary Table 4
Supplementary Information (XLSX 260 kb)
Supplementary Table 5
Supplementary Information (XLSX 13 kb)
Supplementary Table 6
Supplementary Information (XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Calvo, F., Ege, N., Grande-Garcia, A. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15, 637–646 (2013). https://doi.org/10.1038/ncb2756
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2756
This article is cited by
-
PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9
Cell Death & Disease (2023)
-
Targeting tumor-stroma communication by blocking endothelin-1 receptors sensitizes high-grade serous ovarian cancer to PARP inhibition
Cell Death & Disease (2023)
-
Carcinoma-associated fibroblast-derived lysyl oxidase-rich extracellular vesicles mediate collagen crosslinking and promote epithelial-mesenchymal transition via p-FAK/p-paxillin/YAP signaling
International Journal of Oral Science (2023)
-
Biology, vulnerabilities and clinical applications of circulating tumour cells
Nature Reviews Cancer (2023)
-
Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment
Nature Communications (2023)