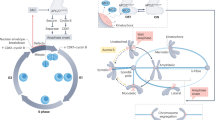
Abstract
The spindle assembly checkpoint is a conserved signalling pathway that protects genome integrity. Given its central importance, this checkpoint should withstand stochastic fluctuations and environmental perturbations, but the extent of and mechanisms underlying its robustness remain unknown. We probed spindle assembly checkpoint signalling by modulating checkpoint protein abundance and nutrient conditions in fission yeast. For core checkpoint proteins, a mere 20% reduction can suffice to impair signalling, revealing a surprising fragility. Quantification of protein abundance in single cells showed little variability (noise) of critical proteins, explaining why the checkpoint normally functions reliably. Checkpoint-mediated stoichiometric inhibition of the anaphase activator Cdc20 (Slp1 in Schizosaccharomyces pombe) can account for the tolerance towards small fluctuations in protein abundance and explains our observation that some perturbations lead to non-genetic variation in the checkpoint response. Our work highlights low gene expression noise as an important determinant of reliable checkpoint signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Barkai, N. & Shilo, B. Z. Variability and robustness in biomolecular systems. Mol. Cell 28, 755–760 (2007).
Raj, A. & van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226 (2008).
Lara-Gonzalez, P., Westhorpe, F. G. & Taylor, S. S. The spindle assembly checkpoint. Curr. Biol. 22, R966–R980 (2012).
Jia, L., Kim, S. & Yu, H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem. Sci. 38, 302–311 (2013).
Vleugel, M., Hoogendoorn, E., Snel, B. & Kops, G. J. Evolution and function of the mitotic checkpoint. Dev. Cell 23, 239–250 (2012).
Pines, J. Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12, 427–438 (2011).
Primorac, I. & Musacchio, A. Panta rhei: the APC/C at steady state. J. Cell Biol. 201, 177–189 (2013).
Sironi, L. et al. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).
De Antoni, A. et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005).
Mapelli, M. & Musacchio, A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr. Opin. Struct. Biol. 17, 716–725 (2007).
Luo, X. & Yu, H. Protein metamorphosis: the two-state behavior of Mad2. Structure 16, 1616–1625 (2008).
Chao, W. C., Kulkarni, K., Zhang, Z., Kong, E. H. & Barford, D. Structure of the mitotic checkpoint complex. Nature 484, 208–213 (2012).
Doncic, A., Ben-Jacob, E. & Barkai, N. Noise resistance in the spindle assembly checkpoint. Mol. Syst. Biol. 2, 1–6 (2006).
Wu, J. Q. & Pollard, T. D. Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310–314 (2005).
Ohi, M. D. et al. Structural organization of the anaphase-promoting complex bound to the mitotic activator Slp1. Mol. Cell 28, 871–885 (2007).
Yamashita, Y. M. et al. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature 384, 276–279 (1996).
Vanoosthuyse, V., Valsdottir, R., Javerzat, J. P. & Hardwick, K. G. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell Biol. 24, 9786–9801 (2004).
He, X., Jones, M. H., Winey, M. & Sazer, S. Mph1, a member of the Mps1-like family of dual specificity protein kinases, is required for the spindle checkpoint in S. pombe. J. Cell Sci. 111, 1635–1647 (1998).
Zich, J. et al. Kinase activity of fission yeast Mph1 is required for Mad2 andMad3 to stably bind the anaphase promoting complex. Curr. Biol. 22, 296–301 (2012).
Hiraoka, Y., Toda, T. & Yanagida, M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell 39, 349–358 (1984).
Millband, D. N. & Hardwick, K. G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell Biol. 22, 2728–2742 (2002).
Bar-Even, A. et al. Noise in protein expression scales with natural protein abundance. Nat. Genet. 38, 636–643 (2006).
Newman, J. R. et al. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441, 840–846 (2006).
Marguerat, S. et al. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell 151, 671–683 (2012).
Amorim, M. J., Cotobal, C., Duncan, C. & Mata, J. Global coordination of transcriptional control and mRNA decay during cellular differentiation. Mol. Syst. Biol. 6, 380 (2010).
Sun, M. et al. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 22, 1350–1359 (2012).
Castelnuovo, M. et al. Bimodal expression of PHO84 is modulated by early termination of antisense transcription. Nat. Struct. Mol. Biol. 20, 851–858 (2013).
Emre, D., Terracol, R., Poncet, A., Rahmani, Z. & Karess, R. E. A mitotic role for Mad1 beyond the spindle checkpoint. J. Cell Sci. 124, 1664–1671 (2011).
Chung, E. & Chen, R. H. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell 13, 1501–1511 (2002).
Schuyler, S. C., Wu, Y. F. & Kuan, V. J. The Mad1-Mad2 balancing act—a damaged spindle checkpoint in chromosome instability and cancer. J. Cell Sci. 125, 4197–4206 (2012).
Tipton, A. R. et al. BUBR1 and closed MAD2 (C-MAD2) interact directly to assemble a functional mitotic checkpoint complex. J. Biol. Chem. 286, 21173–21179 (2011).
Yang, M. et al. Insights into mad2 regulation in the spindle checkpoint revealed by the crystal structure of the symmetric mad2 dimer. PLoS Biol. 6, 643–655 (2008).
Heinrich, S., Windecker, H., Hustedt, N. & Hauf, S. Mph1 kinetochore localization is crucial and upstream in the hierarchy of spindle assembly checkpoint protein recruitment to kinetochores. J. Cell Sci. 125, 4720–4727 (2012).
Sironi, L. et al. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 20, 6371–6382 (2001).
Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Barnhart, E. L., Dorer, R. K., Murray, A. W. & Schuyler, S. C. Reduced Mad2 expression keeps relaxed kinetochores from arresting budding yeast in mitosis. Mol. Biol. Cell 22, 2448–2457 (2011).
Michel, L. S. et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409, 355–359 (2001).
Iwanaga, Y. et al. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 67, 160–166 (2007).
Ryan, S. D. et al. Up-regulation of the mitotic checkpoint component Mad1 causes chromosomal instability and resistance to microtubule poisons. Proc. Natl Acad. Sci. USA 109, E2205–E2214 (2012).
Pan, J. & Chen, R. H. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 18, 1439–1451 (2004).
Sczaniecka, M. et al. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C). J. Biol. Chem. 283, 23039–23047 (2008).
Heim, R., Cubitt, A. B. & Tsien, R. Y. Improved green fluorescence. Nature 373, 663–664 (1995).
Buchler, N. E. & Louis, M. Molecular titration and ultrasensitivity in regulatory networks. J. Mol. Biol. 384, 1106–1119 (2008).
Reddy, S. K., Rape, M., Margansky, W. A. & Kirschner, M. W. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 446, 921–925 (2007).
Mansfeld, J., Collin, P., Collins, M. O., Choudhary, J. S. & Pines, J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat. Cell Biol. 13, 1234–1243 (2011).
Uzunova, K. et al. APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex. Nat. Struct. Mol. Biol. 19, 1116–1123 (2012).
Sigal, A. et al. Variability and memory of protein levels in human cells. Nature 444, 643–646 (2006).
Spencer, S. L., Gaudet, S., Albeck, J. G., Burke, J. M. & Sorger, P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432 (2009).
Balazsi, G., van Oudenaarden, A. & Collins, J. J. Cellular decision making and biological noise: from microbes to mammals. Cell 144, 910–925 (2011).
Chen, R. H., Brady, D. M., Smith, D., Murray, A. W. & Hardwick, K. G. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell 10, 2607–2618 (1999).
Fraschini, R. et al. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 20, 6648–6659 (2001).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Shah, J. V. et al. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14, 942–952 (2004).
Poddar, A., Stukenberg, P. T. & Burke, D. J. Two complexes of spindle checkpoint proteins containing Cdc20 and Mad2 assemble during mitosis independently of the kinetochore in Saccharomyces cerevisiae. Eukaryot. Cell 4, 867–878 (2005).
Nilsson, J., Yekezare, M., Minshull, J. & Pines, J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10, 1411–1420 (2008).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Uhlen, M. et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250 (2010).
Baker, D. J. et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744–749 (2004).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008).
Oliva, A. et al. The cell cycle-regulated genes of Schizosaccharomyces pombe. PLoS Biol. 3, 1239–1260 (2005).
Rustici, G. et al. Periodic gene expression program of the fission yeast cell cycle. Nat. Genet. 36, 809–817 (2004).
Peng, X. et al. Identification of cell cycle-regulated genes in fission yeast. Mol. Biol. Cell 16, 1026–1042 (2005).
Bahler, J. et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 (1998).
Rosenow, M. A., Huffman, H. A., Phail, M. E. & Wachter, R. M. The crystal structure of the Y66L variant of green fluorescent protein supports a cyclization-oxidation-dehydration mechanism for chromophore maturation. Biochemistry 43, 4464–4472 (2004).
Matsuyama, A. et al. pDUAL, a multipurpose, multicopy vector capable of chromosomal integration in fission yeast. Yeast 21, 1289–1305 (2004).
Russell, P. & Nurse, P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145–153 (1986).
Hagan, I. & Yanagida, M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 347, 563–566 (1990).
Yokobayashi, S. & Watanabe, Y. The kinetochore protein Moa1 enables cohesion-mediated monopolar attachment at meiosis I. Cell 123, 803–817 (2005).
Windecker, H., Langegger, M., Heinrich, S. & Hauf, S. Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin. EMBO Rep. 10, 1022–1028 (2009).
He, X., Patterson, T. E. & Sazer, S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl Acad. Sci. USA 94, 7965–7970 (1997).
Tange, Y. & Niwa, O. Novel mad2 alleles isolated in a Schizosaccharomyces pombe gamma-tubulin mutant are defective in metaphase arrest activity, but remain functional for chromosome stability in unperturbed mitosis. Genetics 175, 1571–1584 (2007).
Krien, M. J. et al. A NIMA homologue promotes chromatin condensation in fission yeast. J. Cell Sci. 111, 967–976 (1998).
Grallert, A. & Hagan, I. M. Schizosaccharomyces pombe NIMA-related kinase, Fin1, regulates spindle formation and an affinity of Polo for the SPB. EMBO J. 21, 3096–3107 (2002).
Funabiki, H., Kumada, K. & Yanagida, M. Fission yeast Cut1 and Cut2 are essential for sister chromatid separation, concentrate along the metaphase spindle and form large complexes. EMBO J. 15, 6617–6628 (1996).
Matsumura, T. et al. A brute force postgenome approach to identify temperature-sensitive mutations that negatively interact with separase and securin plasmids. Genes. Cells 8, 341–355 (2003).
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 (1991).
Johnston, K. et al. Vertebrate kinetochore protein architecture: protein copy number. J. Cell Biol. 189, 937–943 (2010).
Widmer, C. et al. GRED: graph-regularized 3D shape reconstruction from highly anisotropic and noisy images. Preprint at http://arXiv.org/abs/1309.4426 (2013).
Neumann, F. R. & Nurse, P. Nuclear size control in fission yeast. J. Cell Biol. 179, 593–600 (2007).
Capoulade, J., Wachsmuth, M., Hufnagel, L. & Knop, M. Quantitative fluorescence imaging of protein diffusion and interaction in living cells. Nat. Biotechnol. 29, 835–839 (2011).
Schmidt, U. et al. Assembly and mobility of exon-exon junction complexes in living cells. RNA 15, 862–876 (2009).
Wu, Y., Genton, M. G. & Stefanski, L. A. A multivariate two-sample mean test for small sample size and missing data. Biometrics 62, 877–885 (2006).
Mueller, F. et al. FISH-quant: automatic counting of transcripts in 3D FISH images. Nat. Methods 10, 277–278 (2013).
Perkins, W., Tygert, M. & Ward, R. Chi-square and classical exact tests often wildly misreport significance; the remedy lies in computers. Preprint at http://arXiv.org/abs/1108.4126 (2011).
Yamada, H. Y., Matsumoto, S. & Matsumoto, T. High dosage expression of a zinc finger protein, Grt1, suppresses a mutant of fission yeast slp1(+), a homolog of CDC20/p55CDC/Fizzy. J. Cell Sci. 113, 3989–3999 (2000).
Acknowledgements
We thank S. Umrania and G. Raetsch for help with developing image analysis software, M. Wachsmuth for FCS analysis software, D. Zenklusen and S-R. Imrazene for sharing their FISH protocol, B. Schwalb and A. Tresch for providing mRNA half-life measurements, N. Hustedt and J. Sauerwald for experiments, E. Illgen, E. Schwoerzer, J. Binder, F. Bolukbasi and W. Hauf for excellent technical help, T. Holder for data processing scripts, C. Liebig for advice on image processing, and F. Theis for support in developing the multi-experiment modelling. We are grateful to K. Gull and T. Matsumoto for antibodies, and to F. Bono, F. Herzog, M. Hothorn, S. Legewie and Y. Watanabe for comments on the manuscript. This work was supported by the Max Planck Society (S. Hauf, S. Heinrich, J.K., C.W. and P.D.), the Ernst Schering Foundation (fellowship to S. Heinrich), the Boehringer Ingelheim Fonds (fellowship to J.K.), the Memorial Sloan-Kettering Cancer Center (C.W. and P.D.), the Cluster of Excellence in Simulation Technology (EXC 310) of the German Research Foundation (DFG; N.R.), the Human Frontier Science Program (HFSP; postdoctoral fellowship to S.T.), the funding program for junior professors of the Ministry of Science, Research and Arts of Baden-Württemberg (E-M.G. and N.R.), and the Federal Ministry of Education and Research (BMBF) within the Virtual Liver project (Grant No. 0315766; J.H.).
Author information
Authors and Affiliations
Contributions
S. Heinrich designed and performed all experiments, with the exception of FCS (by S.T. and M.K.), Slp1 quantification in EMM (J.K.), and characterization of the Mad1-RL/AG mutant (J.K.); S. Heinrich constructed plasmids and strains with contributions by J.K.; E-M.G., N.R., J.H. and S. Hauf performed modelling and statistical evaluation; C.W. developed the automated nuclear tracking approach with contributions from P.D.; S. Hauf devised the project and wrote the manuscript together with S. Heinrich and input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Functionality of GFP fusion proteins.
a Strains with SAC protein-GFP fusions have a functional SAC. Cells were either grown at permissive temperature for the tubulin mutant nda3-KM311 (30 °C; cyc; cycling cells) or at restrictive temperature (18 °C; arr.; cells arrested in mitosis) (n>100 cells for each strain and condition). Plo1 localization to the spindle pole body (Plo1 signal) indicated that cells were in mitosis. Localization of the SAC protein-GFP fusions to kinetochores was additionally scored (GFP signal). Shown is one representative out of two independent experiments. b Strains expressing GFP-tagged SAC components grew similar to wild type (WT), with the exception of strains expressing bub3+-GFP and mph1+-GFP, whose growth was impaired on benomyl-containing medium. A serial dilution of cells was spotted and grown at the indicated temperatures on rich medium or rich medium supplemented with 8 μg/ml of the microtubule drug benomyl. c Strains expressing GFP-tagged SAC components were crossed to strains containing mutations that are known to cause a synthetic growth defect when combined with the respective SAC gene deletion. A growth assay of tetrads resulting from these crosses was performed as in (b). Except for the mad1+-GFP gtb1-93 and apc5+-GFP cut2-364 double mutant, which had slightly impaired growth, none of the double mutants showed a synthetic growth defect.
Supplementary Figure 2 Imaging conditions for quantification by WiFDeM.
a–c Microscope and camera respond linearly to signals in the relevant range. Protein extracts of wild type cells were mixed with serial dilutions of either recombinant GFP (rGFP; 3:4 dilutions starting from 200 nM) or Alexa-488 coupled antibodies (3:4 dilutions starting from 500 ng/mL). Two independent serial dilutions were imaged (dilution 1 and dilution 2) with different neutral density filters (ND) and different exposure times (0.25 s and 0.5 s). d Schematic representation of the quantitative imaging procedure. Cells containing GFP-labelled SAC proteins and cells without GFP (wild type; WT) were mixed and loaded into a microfluidics cell-trapping device. Cells were constantly supplied with fresh medium throughout the imaging process. Image stacks for GFP and mCherry fluorescence were acquired, and a DIC image was taken from the middle of the stack. Uneven illumination of the images was corrected by flatfielding and image stacks were deconvolved. 20 planes of the imaged stack were either used directly for 3D nuclear segmentation or sum-projected to a single plane and fused to the DIC image for 2D cellular segmentation. For 3D nuclear segmentation, the nuclear rim localization of Cut11-mCherry was used as marker. For 2D segmentation, the cellular outline in the DIC image was used and was converted to an estimate of cellular volume. e Representative single plane images and sum projections. To differentiate cells with a very weak GFP signal (Apc5-GFP, Apc15-GFP or Mph1-GFP) from cells that do not express GFP (wild type; WT), wild type cells in these cell mixtures expressed the membrane protein Gpi16-mCherry in addition to the nuclear marker Cut11-mCherry, as shown on the right side.
Supplementary Figure 3 Absolute quantification of SAC proteins and APC/C subunits.
a,b Quantification of free GFP, Apc15-GFP and Mad3-GFP by fluorescence correlation spectroscopy (FCS). Fluorescence fluctuations were determined in interphase nuclei. Shown in (a) are representative auto-correlation curves and the corresponding fit. The amplitude,  of the autocorrelation curve is inversely proportional to the concentration of the fluorescent protein. The frequency distributions for the nuclear concentration in single cells are shown in (b). c Mad3-GFP and Apc15-GFP are excluded from the nucleolus (arrows), but free GFP is not. A single plane of representative nuclei in interphase is shown. GFP signals are differently scaled for different proteins to achieve good visibility. d The nucleolus occupies about ∼ 18% of the nuclear volume. The nucleolar volume was determined by segmentation of either Nuc1-GFP, which localizes to the nucleolus, or by segmentation of the region from which Mad3-GFP is excluded. (n = 10 cells (Nuc1-GFP), n = 9 and 11 cells (Mad3-GFP); error bars = s.d.). e Nuclear concentrations were measured by FCS outside the nucleolus (grey, data from (b)). The average concentration in the nucleus (blue) was calculated using the nucleolar volume determined in (d). (Error bars = s.d.) f Quantification of free GFP by quantitative immunoblotting. Cdc25-22 cells expressing Pmad3–GFP were arrested before mitosis in rich medium. Three technical replicates were harvested at the indicated time points after release and were analysed by immunoblotting using anti-GFP and anti-Cdc2 (loading control) antibodies. Recombinant GFP (rGFP, Clontech) was mixed with an extract from G2-arrested cells not expressing Pmad3–GFP as standard for quantification. The graph on the right shows the average concentration from 4 independent experiments, of which one is shown on the left. (Error bars = s.d.; n = 4 experiments) g Equal detection of wtGFP and S65T-GFP with anti-GFP antibody. We used recombinant wild type GFP (wtGFP) for quantification of S65T-GFP-tagged checkpoint proteins (f). To confirm that the anti-GFP antibody detected wtGFP and S65T-GFP equally well, Mad3 was tagged with either wtGFP or S65T-GFP. Two independent strains (labelled 1 and 2) were compared by immunoblotting using anti-GFP and anti-Cdc2 (loading control) antibodies. Percentages on top of each lane indicate how much of the original extract was loaded. h Incomplete maturation of GFP is unlikely to account for the difference in GFP concentration determined by FCS and immunoblotting. We arrested cells in mitosis by the microtubule drug MBC and additionally treated with cycloheximide to block protein synthesis. GFP, but not Slp1, was stable for 60 min under these conditions. This indicates that protein turnover of GFP is low and that most of the GFP present at any given moment should have had enough time to form the fluorophore. In addition, GFP-tagged Mad3 as well as untagged Mad2 were similarly stable as GFP in cycloheximide-treated cells, indicating low turn-over of these SAC proteins. (*, antibody cross-reaction).
of the autocorrelation curve is inversely proportional to the concentration of the fluorescent protein. The frequency distributions for the nuclear concentration in single cells are shown in (b). c Mad3-GFP and Apc15-GFP are excluded from the nucleolus (arrows), but free GFP is not. A single plane of representative nuclei in interphase is shown. GFP signals are differently scaled for different proteins to achieve good visibility. d The nucleolus occupies about ∼ 18% of the nuclear volume. The nucleolar volume was determined by segmentation of either Nuc1-GFP, which localizes to the nucleolus, or by segmentation of the region from which Mad3-GFP is excluded. (n = 10 cells (Nuc1-GFP), n = 9 and 11 cells (Mad3-GFP); error bars = s.d.). e Nuclear concentrations were measured by FCS outside the nucleolus (grey, data from (b)). The average concentration in the nucleus (blue) was calculated using the nucleolar volume determined in (d). (Error bars = s.d.) f Quantification of free GFP by quantitative immunoblotting. Cdc25-22 cells expressing Pmad3–GFP were arrested before mitosis in rich medium. Three technical replicates were harvested at the indicated time points after release and were analysed by immunoblotting using anti-GFP and anti-Cdc2 (loading control) antibodies. Recombinant GFP (rGFP, Clontech) was mixed with an extract from G2-arrested cells not expressing Pmad3–GFP as standard for quantification. The graph on the right shows the average concentration from 4 independent experiments, of which one is shown on the left. (Error bars = s.d.; n = 4 experiments) g Equal detection of wtGFP and S65T-GFP with anti-GFP antibody. We used recombinant wild type GFP (wtGFP) for quantification of S65T-GFP-tagged checkpoint proteins (f). To confirm that the anti-GFP antibody detected wtGFP and S65T-GFP equally well, Mad3 was tagged with either wtGFP or S65T-GFP. Two independent strains (labelled 1 and 2) were compared by immunoblotting using anti-GFP and anti-Cdc2 (loading control) antibodies. Percentages on top of each lane indicate how much of the original extract was loaded. h Incomplete maturation of GFP is unlikely to account for the difference in GFP concentration determined by FCS and immunoblotting. We arrested cells in mitosis by the microtubule drug MBC and additionally treated with cycloheximide to block protein synthesis. GFP, but not Slp1, was stable for 60 min under these conditions. This indicates that protein turnover of GFP is low and that most of the GFP present at any given moment should have had enough time to form the fluorophore. In addition, GFP-tagged Mad3 as well as untagged Mad2 were similarly stable as GFP in cycloheximide-treated cells, indicating low turn-over of these SAC proteins. (*, antibody cross-reaction).
Supplementary Figure 4 Abundance of Mad1-, Mad2- and Mad3-GFP after promoter modifications.
Extracts from asynchronously growing cultures in rich medium were analysed by immunoblotting using anti-GFP, anti-Mad1, anti-Mad2 and either anti-Cdc2 or anti-tubulin antibodies (as loading controls). Mad1-GFP strains are shown in (a), Mad2-GFP strains in (b) and Mad3-GFP strains in (c). Percentages on top of each lane indicate how much of the original extract was loaded. Percentages in purple indicate the estimated protein abundance compared to wild type (WT). Dashed boxes indicate bands with similar signal strength from which protein abundances of the promoter-modified strains were deduced. Estimations of the abundance relative to wild type are typically based on several experiments, of which only one representative experiment is shown. (s.e. = short exposure, l.e. = long exposure, Pmad3 length = length of the remaining mad3 promoter; see Supplementary Table 3 for the molecular changes in the promoter region).
Supplementary Figure 5 Analysis of the Mad1–RL/AG mutant as well as Mad1 and Mad2 overexpression.
a Mad2-mCherry does not co-localise with Mad1 that contains two point mutations (CRVLQHRS to CAVGQHRS) in the Mad2-binding site (Mad1–RL/AG). Representative images from asynchronous cultures are shown. Scale bar: 10 μm. b Mad1–RL/AG is present at similar levels as wild type Mad1, and Mad2 abundance is unaffected. Extracts from asynchronously growing cultures in rich medium were analysed by immunoblotting using anti-Mad1, anti-Mad2 and anti-Cdc2 (as loading control) antibodies. Percentages on top of each lane indicate how much of the original extract was loaded. The asterisk indicates a cross-reacting band. c Input and flow-through of the immunoprecipitation shown in Fig. 4a. Extracts from asynchronously growing cultures in rich medium were used for immunoprecipitation (IP) of Mad1–RL/AG or Mad1-GFP (expressed to 300%) using anti-Mad1 antibodies. Shown are immunoblots of the extract used for the IP (input) and of the flow through after immunoprecipitation (FT after IP). d High immunoprecipitation efficiency for Mad1 or Mad1-GFP. Extracts from asynchronously growing cultures in rich medium were used for immunoprecipitation of Mad1 or Mad1-GFP using anti-Mad1 antibodies and analysed for the amount of Mad1 remaining in the extract after IP (FT (flow through) after IP). e 50 % additional Mad2 increases free Mad2 in cells with 300% Mad1-GFP. Extracts from asynchronously growing cultures in rich medium were used for immunoprecipitation of Mad1 using anti-Mad1 antibodies and analysed for co-immunoprecipitation of Mad2. The input and FT is 6.25% of the amount used for the IP sample. Mad1 was largely depleted from the flow through after IP. Quantifications of the flow through are shown on the right (see Methods). The depletion of free Mad2 by increasing the Mad1 abundance to 300 % is not as strong as could be expected (Supplementary Note (B1)). Shown is one representative out of two independent experiments. f Mad1 and Mad2 abundance in the strains shown in Fig. 4b. Extracts from asynchronously growing cultures in rich medium were analysed by immunoblotting using anti-Mad1 (for Mad1 and Mad1-GFP detection), anti-GFP (for Mad2-GFP detection), anti-Mad2 (for Mad2 detection) and anti-Cdc2 antibodies (as loading control). The asterisk indicates a cross-reacting band. g Addition of 50 % untagged Mad2 rescues the checkpoint defect in cells with 300 % Mad1, similar to addition of 50 % Mad2-GFP (Fig. 4b). To determine SAC activity, cells were followed by live cell imaging at 16 °C as in Fig. 2 (left side). Extracts from asynchronously growing cultures from the same strains were analysed by immunoblotting (right side) using anti-Mad1, anti-Mad2 and anti-Cdc2 antibodies (as loading control). Shown is one representative out of two independent experiments. h Cells with 200 % Mad2-GFP stay in mitosis for a similar time as cells with 100% Mad2-GFP. Cells were cultured in rich medium and followed by live cell imaging at 30 °C. The time in mitosis was determined from SPB separation to spindle elongation using Plo1-mCherry as marker for the SPBs. Each circle represents one cell. Shown in purple are mean and s.d. i 200% Mad2-GFP cannot overcome the checkpoint defect of a mad1 deletion. nda3-KM311 strains were followed by live cell imaging at 16 °C and the time in mitosis was scored as in Fig. 2 (left side). Extracts from asynchronously growing cultures from the same strains were analysed by immunoblotting (right side) using anti-Mad1, anti-GFP and anti-Cdc2 antibodies (as loading control).
Supplementary Figure 6 Analysis of Mad2 abundance at kinetochores, in the nucleoplasm and in complex with Mad1.
a Abundance of 40 % and 20 % Mad2 in the nucleoplasm and at kinetochores. The amount of Mad2-GFP in the indicated strains was followed as cells entered mitosis in the absence of microtubules. (Error bars = s.d.; n = 41/38/29 cells for 100/40/20 % Mad2). We tested for similarity of the curves by pooled component test. The differences between strains for signals both at the kinetochore and in the nucleoplasm were statistically significant (p<0.05). b Reduction of Mad2 to 20 % reduces the Mad1-bound and the free pool of Mad2. Extracts from asynchronously growing cultures in rich medium were used for immunoprecipitation (IP) of Mad1 using anti-Mad1 antibodies and analysed for co-immunoprecipitation of Mad2. Percentages on top of each lane indicate how much of the original extract or the immunoprecipitation was loaded. The input and flow through (FT) loaded is 15 % of the amount used for the IP sample. Quantifications of the flow through and the IP are shown on the right (see Methods). For 100 % and 40 % Mad2, one representative out of three independent experiments is shown. c Mad2-GFP recruitment to the kinetochore is not decreased in mad3 Δ or slp1-mr63. The amount of Mad2-GFP at the kinetochore was recorded as cells entered mitosis in the absence of microtubules. (Error bars = s.d.) d The Mad2-R133A mutation causes a checkpoint defect. Strains were followed by live cell imaging as in Fig. 2. e Mad2-R133A and abundance-reduced versions are present at similar levels as wild type Mad2. Extracts from asynchronously growing cultures in rich medium were analysed by immunoblotting using anti-Mad1, anti-GFP and anti-Cdc2 (as loading control) antibodies. Percentages on top of each lane indicate how much of the original extract was loaded. f Statistical analysis of Mad2 abundance in the nucleoplasm and at the kinetochore. Intensity curves for the Mad2-GFP and Mad2-R133A-GFP strains, also shown in Fig. 4d, were compared by a pooled component test. The cumulative p-value is plotted in pink. A p-value of 0.05 is shown as dashed red line. g Reduction of Mad1 to 30 % considerably decreases the Mad1 amount at unattached kinetochores. The amount of Mad1-GFP at the kinetochore in the indicated strains was followed as cells entered mitosis in the absence of microtubules (error bars = s.d.).
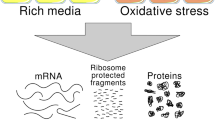
Supplementary Figure 7 Quantification of Slp1 mRNA and protein abundance.
a Nuclear volume increase in cdc25-22 arrest. Cells expressing cut11+-mCherry were shifted for 4.5 hours (YEA) or 5 hours (EMM) to 36 °C for synchronization in G2. Cdc25+ cells were mixed into the G2-arrested cdc25-22 culture and the mixture was mounted on the microscope stage, which was pre-heated to 36 °C. The nuclear rim localization of Cut11-mCherry was used for nuclear segmentation. (a.u. = arbitrary units, error bars = s.d.). b Quantification of Slp1 in minimal medium by quantitative immunoblotting. Cdc25-22 cells expressing either cut7+ or the kinesin mutant cut7-446 were grown in minimal medium (EMM). Cut7-446 at restrictive temperature causes the formation of a monopolar spindle and activation of the spindle assembly checkpoint (SAC). Three technical replicates were harvested from the start of Slp1 expression until maximal abundance was reached and were analysed by immunoblotting using anti-Slp1 and anti-Cdc2 (loading control) antibodies. Recombinant His6-Slp1 (rSlp1) was mixed with an extract from G2-arrested cells and was used as standard for quantification. (short = short exposure, long = long exposure) Shown is one representative out of two (cut7+) or three (cut7-446) independent experiments. c Mitotic index of samples used for Slp1 abundance determination by quantitative immunoblotting. Cells were grown in rich medium (for cut7+ cells; see Fig. 6a) or minimal medium (EMM; for cut7+ and cut-446 cells; see (b) and (e) in this figure). After G2 arrest, cells in rich medium were released into mitosis at 16 °C, cells in minimal medium were released into mitosis at 30 °C. The mitotic index was determined from the percentage of cells showing a localized Plo1-mCherry signal at spindle pole bodies (SPBs). d Slp1-HA was detectable in mitotic cells and was distributed throughout the cell with a slight enrichment in the nucleus. Cells were immunostained for HA and tubulin. Cells expressing bub1+-HA and wild type cells served as specificity controls. The nucleo-cytoplasmic ratio for Slp1-HA was calculated by dividing the mean intensity in the nucleus (nuc.) by the mean intensity in the cytoplasm (cyto.). Background measured in interphase cells was subtracted. (n = 30 cells; ± = s.d.) Scale bar: 5 μm. Shown is one representative out of two independent experiments. e Slp1 is approximately twice as abundant in minimal medium as in rich medium. Slp1 concentrations determined from the time course experiments shown in Fig. 6a and Supplementary Fig. 6b. The graph shows average concentrations from two (cut7+, both rich and minimal medium) or three (cut7-446, minimal medium) independent experiments. (error bars = s.d.) The table below shows the values for cellular Slp1 concentration (cell) determined by immunoblotting and the estimated nuclear concentration (nucl.) based on the measured nucleo-cytoplasmic ratio (see d). f Slp1 mRNA abundance peaks in mitosis. Single molecule FISH was performed on an asynchronous cell culture grown in minimal medium with probes against Slp1 mRNA. A representative image is shown on the left (scale bar: 5 μm). Plo1-GFP indicates cells in prometaphase. The histogram on the right depicts the mRNA frequency distribution in this sample. (n = 186 cells).
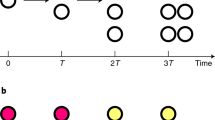
Supplementary Figure 8 Statistical analysis of distribution of the mitosis times, and protein abundance measurements in the two subpopulations.
a Distribution of mitosis times assessed by multi-experiment modelling (Supplementary Note). Mitosis times measured in strains with changed SAC protein abundance shown in Fig. 2 were analysed by multi-experiment modelling for the occurrence of up to two subpopulations. More plausible models have a lower Bayesian information criterion (BIC), and ranking of the models according to their BIC is shown. b In rich medium, no significant difference in Mad2 abundance was observed between the subpopulations. Strains were followed by live cell imaging as in Fig. 2 in either rich or minimal (min.) medium (left side). Mad2-GFP signals were quantified in each population (A and B) as cells entered mitosis (right side) (a.u. = arbitrary units; error bars = s.d.; n = 23/18 cells for population A/B in rich medium; n = 24/32 cells for population A/B in minimal medium). Intensity curves for population A and B were compared by a pooled component test. The cumulative p-value is plotted in grey. For rich medium, one representative out of two independent experiments is shown. c Combined Mad2 and Mad3 abundance are similar between population A and B. Strains expressing both Mad2- and Mad3-GFP in the indicated abundances were analysed as in (b) (a.u. = arbitrary units; error bars = s.d.). d Intensity curves for population A and B from Fig. 8d were compared by a pooled component test. The cumulative p-value is plotted in grey. A p-value <0.05 is considered significant (dashed red line). Mad3 abundance in minimal medium is different between populations A and B. e Bub1-GFP signal intensity in a strain containing non-fluorescent 30 % Mad3-GFP-Y66L was analysed as in (b). (‘no GFP’ = 30 % Mad3-GFP-Y66L without Bub1-GFP; error bars = s.d.; n = 19 cells (no GFP), n = 24 cells (population A), n = 22 cells (population B)) f Titration of Mad3 abundance in a 65 % Mad2-GFP background does not strongly affect the distribution of cells in the two subpopulations. Cells were followed by live cell imaging as in Fig. 2.
Supplementary Figure 9 Entire membranes of cropped immunoblots.
Blue labels on top indicate the antibody used for detection. Dashed red boxes show which regions of the immunoblot were cropped for the individual figures.
Supplementary information
Supplementary Information
Supplementary Information (PDF 6237 kb)
Supplementary Table 1
Supplementary Information (XLSX 40 kb)
Supplementary Table 2
Supplementary Information (XLSX 39 kb)
Supplementary Table 3
Supplementary Information (XLSX 26 kb)
Supplementary Table 4
Supplementary Information (XLSX 30 kb)
Supplementary Table 5
Supplementary Information (XLSX 34 kb)
Supplementary Data
Supplementary Information (ZIP 50881 kb)
Rights and permissions
About this article
Cite this article
Heinrich, S., Geissen, EM., Kamenz, J. et al. Determinants of robustness in spindle assembly checkpoint signalling. Nat Cell Biol 15, 1328–1339 (2013). https://doi.org/10.1038/ncb2864
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2864
This article is cited by
-
MAPK-dependent control of mitotic progression in S. pombe
BMC Biology (2024)
-
Principles and dynamics of spindle assembly checkpoint signalling
Nature Reviews Molecular Cell Biology (2023)
-
Chromosome-associated RNA–protein complexes promote pairing of homologous chromosomes during meiosis in Schizosaccharomyces pombe
Nature Communications (2019)
-
Mitochondrial origins of fractional control in regulated cell death
Nature Communications (2019)
-
The Bub1–Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation
Nature Communications (2016)