Abstract
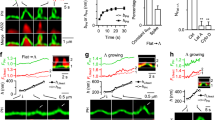
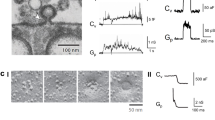
During the process of clathrin-mediated endocytosis an essentially planar area of membrane has to undergo a gross deformation to form a spherical bud. Three ways have been recognized by which membranes can be induced to transform themselves locally from a planar state to one of high curvature: a change in lipid distribution between the leaflets, insertion of a protein into one leaflet and formation of a protein scaffold over the surface1. Such a scaffold is spontaneously generated by clathrin2,3,4,5. Conjectures that the attachment of clathrin was the cause of the change in curvature were challenged on theoretical grounds6, and also by the discovery of a number of clathrin-associated proteins with the capacity to induce membrane curvature7,8,9. We have now developed a cell-free system that has enabled us to demonstrate that clathrin polymerization alone is sufficient to generate spherical buds in a membrane. This process is reversible, as shown by the reassimilation of the buds into the planar membrane when the intra-clathrin contacts are dissociated by the chaperone Hsc70. We further show that the final step in the formation of coated vesicles ensues when clathrin-coated buds are released through the action of dynamin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zimmerberg, J. & Kozlov, M. M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9–19 (2006).
Pearse, B. M. Coated vesicles from pig brain: purification and biochemical characterization. J. Mol. Biol. 97, 93–98 (1975).
Woodward, M. P. & Roth, T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc. Natl Acad. Sci. USA 75, 4394–4398 (1978).
Ungewickell, E. & Branton, D. Assembly units of clathrin coats. Nature 289, 420–422 (1981).
Kirchhausen, T. & Harrison, S. C. Protein organization in clathrin trimers. Cell 23, 755–761 (1981).
Nossal, R. Energetics of clathrin basket assembly. Traffic 2, 138–147 (2001).
Henne, W. M. et al. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure 15, 839–852 (2007).
Gallop, J. L. et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 25, 2898–2910 (2006).
Ford, M. G. et al. Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366 (2002).
Drake, M. T. & Traub, L. M. Interaction of two structurally distinct sequence types with the clathrin terminal domain beta-propeller. J. Biol. Chem. 276, 28700–28709 (2001).
Chen, H. et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793–797 (1998).
Kalthoff, C., Alves, J., Urbanke, C., Knorr, R. & Ungewickell, E. J. Unusual structural organization of the endocytic proteins AP180 and epsin 1. J. Biol. Chem. 277, 8209–8216 (2002).
Chebotareva, N. A., Kurganov, B. I. & Livanova, N. B. Biochemical effects of molecular crowding. Biochemistry 69, 1239–1251 (2004).
Lindner, R. & Ungewickell, E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 267, 16567–16573 (1992).
Morgan, J. R., Prasad, K., Hao, W., Augustine, G. J. & Lafer, E. M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 20, 8667–8676 (2000).
Ford, M. G. et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 (2001).
Norris, F. A., Ungewickell, E. & Majerus, P. W. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP- 3/AP180) and inhibits clathrin cage assembly in vitro. J. Biol. Chem. 270, 214–217 (1995).
McMahon, H. T. & Gallop, J. L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 (2005).
Lata, S., Reichel, A., Brock, R., Tampe, R. & Piehler, J. High-affinity adaptors for switchable recognition of histidine-tagged proteins. J. Am. Chem. Soc. 127, 10205–10215 (2005).
Ungewickell, E. et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature 378, 632–635 (1995).
Traub, L. M. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta 1744, 415–437 (2005).
Semerdjieva, S. et al. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J. Cell Biol. 183, 499–511 (2008).
Nossal, R. & Zimmerberg, J. Endocytosis: curvature to the ENTH degree. Curr. Biol. 12, R770–R772 (2002).
den Otter, W. K. & Briels, W. J. The generation of curved clathrin coats from flat plaques. Traffic 12, 1407–1416 (2011).
Van der Bliek, A. M. et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122, 553–563 (1993).
Chen, M. S. et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351, 583–586 (1991).
Sweitzer, S. M. & Hinshaw, J. E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93, 1021–1029 (1998).
Ramachandran, R. & Schmid, S. L. Real-time detection reveals that effectors couple dynamin’s GTP-dependent conformational changes to the membrane. EMBO J. 27, 27–37 (2008).
Pucadyil, T. J. & Schmid, S. L. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135, 1263–1275 (2008).
Schlossman, D. M., Schmid, S. L., Braell, W. A. & Rothman, J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J. Cell Biol. 99, 723–733 (1984).
Ahle, S., Mann, A., Eichelsbacher, U. & Ungewickell, E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 7, 919–929 (1988).
Keen, J. H., Willingham, M. C. & Pastan, I. Clathrin coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell 16, 303–312 (1979).
Higgins, M. K. & McMahon, H. T. In vitro reconstitution of discrete stages of dynamin-dependent endocytosis. Methods Enzymol. 404, 597–611 (2005).
Roux, A., Uyhazi, K., Frost, A. & De Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 (2006).
Scheele, U. et al. Molecular and functional characterization of clathrin- and AP-2-binding determinants within a disordered domain of auxilin. J. Biol. Chem. 278, 25357–25368 (2003).
Meyerholz, A. et al. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic 6, 1225–1234 (2005).
Lindner, R. & Ungewickell, E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 267, 16567–16573 (1992).
Lud, S. Q., Nikolaides, M. G., Haase, I., Fischer, M. & Bausch, A. R. Field effect of screened charges: electrical detection of peptides and proteins by a thin-film resistor. ChemPhysChem 7, 379–384 (2006).
Israelachvili, J. N. & Mitchell, D. J. A model for the packing of lipids in bilayer membranes. Biochim. Biophys. Acta 389, 13–19 (1975).
Ehrlich, M. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Acknowledgements
We thank H. Böning, C. Lemke and H. Ungewickell for expert technical assistance. Special thanks go to W. B. Gratzer and A. Ungewickell for their critical comments on this work. The German Research Foundation (DFG) supported early stages of this study.
Author information
Authors and Affiliations
Contributions
E.J.U. and P.N.D. designed the study, conducted the experiments, interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1217 kb)
Rights and permissions
About this article
Cite this article
Dannhauser, P., Ungewickell, E. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat Cell Biol 14, 634–639 (2012). https://doi.org/10.1038/ncb2478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2478
This article is cited by
-
Generation of nanoscopic membrane curvature for membrane trafficking
Nature Reviews Molecular Cell Biology (2023)
-
The beauty of simplicity in membrane biology
Nature Cell Biology (2022)
-
Actin polymerization promotes invagination of flat clathrin-coated lattices in mammalian cells by pushing at lattice edges
Nature Communications (2022)
-
Cell-free biogenesis of bacterial division proto-rings that can constrict liposomes
Communications Biology (2020)
-
Human FCHO1 deficiency reveals role for clathrin-mediated endocytosis in development and function of T cells
Nature Communications (2020)