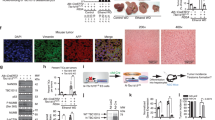
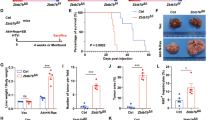
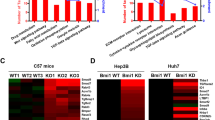
Abstract
Understanding stage-dependent oncogenic mechanisms is critical to develop not only targeted therapies, but also diagnostic markers and preventive strategies. The mechanisms acting during cancer initiation remain elusive, largely owing to a lack of suitable animal models and limited availability of human precancerous lesions. Here we show using genetic mouse models specific for liver cancer initiation, that survival of initiated cancer cells is controlled by c-Jun, independently of p53, through suppressing c-Fos-mediated apoptosis. Mechanistically, c-Fos induces SIRT6 transcription, which represses survivin by reducing histone H3K9 acetylation and NF-κB activation. Importantly, increasing the level of SIRT6 or targeting the anti-apoptotic activity of survivin at the initiation stage markedly impairs cancer development. Moreover, in human dysplastic liver nodules, but not in malignant tumours, a specific expression pattern with increased c-Jun–survivin and attenuated c-Fos–SIRT6 levels was identified. These results reveal a regulatory network connecting stress response and histone modification in liver tumour initiation, which could be targeted to prevent liver tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Change history
14 March 2013
A correction has been made to a sentence in the section "Interfering with liver cancer initiation prevents tumorigenesis". A correction to Fig. 8c and further clarification of Figs 1a, 2b, 3d, 4c and 4g can be found in the accompanying Corrigendum.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Gotay, C. C. Cancer prevention: major initiatives and looking into the future. Exp. Rev. Pharmacoecon Outcomes Res. 10, 143–154 (2010).
Farazi, P. A. & DePinho, R. A. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat. Rev. Cancer 6, 674–687 (2006).
Poon, R. T. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology 54, 757–759 (2011).
He, G. et al. Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17, 286–297 (2010).
Takami, T. et al. Loss of hepatocyte growth factor/c-Met signaling pathway accelerates early stages of N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Res. 67, 9844–9851 (2007).
Maeda, S., Kamata, H., Luo, J. L., Leffert, H. & Karin, M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 (2005).
Eferl, R. & Wagner, E. F. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3, 859–868 (2003).
Lopez-Bergami, P., Lau, E. & Ronai, Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat. Rev. Cancer 10, 65–76 (2010).
Lee, J. S. et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature Med. 12, 410–416 (2006).
Eferl, R. et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell 112, 181–192 (2003).
Hui, L., Zatloukal, K., Scheuch, H., Stepniak, E. & Wagner, E. F. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J. Clin. Invest. 118, 3943–3953 (2008).
Hui, L. et al. p38α suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat. Genet 39, 741–749 (2007).
Das, M., Garlick, D. S., Greiner, D. L. & Davis, R. J. The role of JNK in the development of hepatocellular carcinoma. Gene. Dev. 25, 634–645 (2011).
Hasselblatt, P., Rath, M., Komnenovic, V., Zatloukal, K. & Wagner, E. F. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc. Natl Acad. Sci. USA 104, 17105–17110 (2007).
Liu, P. et al. Activation of NF-κB, AP-1 and STAT transcription factors is afrequent and early event in human hepatocellular carcinomas. J. Hepatol. 37, 63–71 (2002).
Fausto, N. & Campbell, J. S. Mouse models of hepatocellular carcinoma. Semin. Liver Dis. 30, 87–98 (2010).
Kellendonk, C., Opherk, C., Anlag, K., Schutz, G. & Tronche, F. Hepatocyte-specific expression of Cre recombinase. Genesis 26, 151–153 (2000).
Ramachandran, R. & Kakar, S. Histological patterns in drug-induced liver disease. J. Clin. Pathol. 62, 481–492 (2009).
Altieri, D. C. Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer 8, 61–70 (2008).
Kinoshita, K. et al. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 56, 706–714 (2007).
Mesri, M., Wall, N. R., Li, J., Kim, R. W. & Altieri, D. C. Cancer gene therapy using a survivin mutant adenovirus. J. Clin. Invest. 108, 981–990 (2001).
Wang, R. H. et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, 11–20 (2008).
Kawahara, T. L. et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136, 62–74 (2009).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Bozec, A. et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 454, 221–225 (2008).
Mehic, D., Bakiri, L., Ghannadan, M., Wagner, E. F. & Tschachler, E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J. Invest. Dermatol. 124, 212–220 (2005).
Goto, H. et al. Complex formation of Plk1 and INCENP required for metaphase–anaphase transition. Nat. Cell Biol. 8, 180–187 (2006).
Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49 658–664 (2009).
Reed, K. R. et al. A limited role for p53 in modulating the immediate phenotype of Apc loss in the intestine. BMC Cancer 8, 162 (2008).
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006).
Feldser, D. M. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Junttila, M. R. et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature 468, 567–571 (2010).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Meixner, A., Karreth, F., Kenner, L., Penninger, J. M. & Wagner, E. F. Jun and JunD-dependent functions in cell proliferation and stress response. Cell. Death Differ. (2010).
Mikula, M. et al. The proto-oncoprotein c-Fos negatively regulates hepatocellular tumorigenesis. Oncogene 22, 6725–6738 (2003).
Fleischmann, A., Jochum, W., Eferl, R., Witowsky, J. & Wagner, E. F. Rhabdomyosarcoma development in mice lacking Trp53 and Fos: tumor suppression by the Fos protooncogene. Cancer Cell 4, 477–482 (2003).
Montorsi, M. et al. Survivin gene expression in chronic liver disease and hepatocellular carcinoma. Hepatogastroenterology 54, 2040–2044 (2007).
Kim, H. S. et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12, 224–236 (2010).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Acknowledgements
This work was initiated at the Research Institute of Molecular Pathology, which is funded by Boehringer Ingelheim. We thank S. Ji for technical support and R. Eferl, P. Hasselblatt, M. Sibilia, P. Angel and J. Hess for critical reading of the manuscript. We are also grateful to D. Altieri (University of Massachusetts, USA) for survivin and SurT34A adenoviruses, K. Chua (Stanford University, USA) for SIRT6 and SIRT6HY plasmids, M. F. Rajewsky (University of Essen, Germany) for antibodies against DNA adducts, and the Biobank of the Medical University of Graz, Austria for providing further human liver samples. Work in the L.H. laboratory is funded by the National Science Foundation of China (31071238), the Ministry of Science and Technology of China (2012CB945001, 2011ZX09307-302-01) and the Chinese Academy of Sciences (the Hundred Talents Program). Work in the E.F.W. laboratory is supported by a grant from F-BBVA and an ERC-Advanced grant ERC-FCK/2008/37. K.Z. is supported by the Austrian genome program GEN-AU.
Author information
Authors and Affiliations
Contributions
L.H., L.B. and E.F.W. designed the project and L.H. analysed early stages of cell death. L.M. characterized the role of survivin, SIRT6 and c-Fos in liver tumour initiation. L.M., Y.J., L.C., L.Q., L.B. and K.Z. analysed human samples. X.C., Z.Q., J.C., Z.H. and H.S. provided technical support on immunohistochemical staining and gene expression analysis. L.H., L.B. and E.F.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 629 kb)
Supplementary Tables 1–9
Supplementary Information (XLS 69 kb)
Rights and permissions
About this article
Cite this article
Min, L., Ji, Y., Bakiri, L. et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol 14, 1203–1211 (2012). https://doi.org/10.1038/ncb2590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2590
This article is cited by
-
ZBTB7B is a permissive regulator of hepatocellular carcinoma initiation by repressing c-Jun expression and function
Cell Death & Disease (2024)
-
Sirt6 ablation in the liver causes fatty liver that increases cancer risky by upregulating Serpina12
EMBO Reports (2024)
-
Dihydroartemisinin imposes positive and negative regulation on Treg and plasma cells via direct interaction and activation of c-Fos
Communications Biology (2023)
-
De novo identification of maximally deregulated subnetworks based on multi-omics data with DeRegNet
BMC Bioinformatics (2022)
-
RNA adenosine deaminase (ADAR1) alleviates high-fat diet-induced nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome
Laboratory Investigation (2022)