Abstract
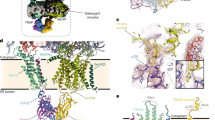
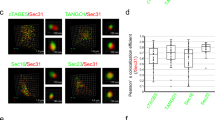
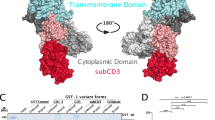
Export of proteins from the endoplasmic reticulum in COPII-coated vesicles occurs at defined sites that contain the scaffolding protein Sec16. We identify TFG-1, a new conserved regulator of protein secretion that interacts directly with SEC-16 and controls the export of cargoes from the endoplasmic reticulum in Caenorhabditis elegans. Hydrodynamic studies indicate that TFG-1 forms hexamers that facilitate the co-assembly of SEC-16 with COPII subunits. Consistent with these findings, TFG-1 depletion leads to a marked decline in both SEC-16 and COPII levels at endoplasmic reticulum exit sites. The sequence encoding the amino terminus of human TFG has been previously identified in chromosome translocation events involving two protein kinases, which created a pair of oncogenes. We propose that fusion of these kinases to TFG relocalizes their activities to endoplasmic reticulum exit sites, where they prematurely phosphorylate substrates during endoplasmic reticulum export. Our findings provide a mechanism by which translocations involving TFG can result in cellular transformation and oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
11 April 2011
In the version of this Article initially published online, the title was incorrect. The error has been corrected in the HTML and PDF versions of the article.
References
Lee, M. C., Miller, E. A., Goldberg, J., Orci, L. & Schekman, R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87–123 (2004).
Bonifacino, J. S. & Glick, B. S. The mechanisms of vesicle budding and fusion. Cell 116, 153–166 (2004).
Bickford, L. C., Mossessova, E. & Goldberg, J. A structural view of the COPII vesicle coat. Curr. Opin. Struct. Biol. 14, 147–153 (2004).
Dancourt, J. & Barlowe, C. Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 79, 777–802 (2010).
Boyadjiev, S. A. et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 38, 1192–1197 (2006).
Lang, M. R., Lapierre, L. A., Frotscher, M., Goldenring, J. R. & Knapik, E. W. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat. Genet. 38, 1198–1203 (2006).
Matsuoka, K. et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263–275 (1998).
Miller, E. A. & Barlowe, C. Regulation of coat assembly—sorting things out at the ER. Curr. Opin. Cell Biol. 22, 447–453 (2010).
Yoshihisa, T., Barlowe, C. & Schekman, R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259, 1466–1468 (1993).
Stagg, S. M. et al. Structure of the Sec13/31 COPII coat cage. Nature 439, 234–238 (2006).
Bi, X., Mancias, J. D. & Goldberg, J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell 13, 635–645 (2007).
Espenshade, P., Gimeno, R. E., Holzmacher, E., Teung, P. & Kaiser, C. A. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J. Cell Biol. 131, 311–323 (1995).
Gimeno, R. E., Espenshade, P. & Kaiser, C. A. COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol. Biol. Cell 7, 1815–1823 (1996).
Shaywitz, D. A., Espenshade, P. J., Gimeno, R. E. & Kaiser, C. A. COPII subunit interactions in the assembly of the vesicle coat. J. Biol. Chem. 272, 25413–25416 (1997).
Whittle, J. R. & Schwartz, T. U. Structure of the Sec13–Sec16 edge element, a template for assembly of the COPII vesicle coat. J. Cell Biol. 190, 347–361 (2010).
Supek, F., Madden, D. T., Hamamoto, S., Orci, L. & Schekman, R. Sec16p potentiates the action of COPII proteins to bud transport vesicles. J. Cell Biol. 158, 1029–1038 (2002).
Li, L. & Xie, T. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605–631 (2005).
Shi, A. et al. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol. Biol. Cell 21, 2930–2943 (2010).
Mallard, F. et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653–664 (2002).
Hughes, H. et al. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J. Cell Sci. 122, 2924–2934 (2009).
Greco, A. et al. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Mol. Cell Biol. 15, 6118–6127 (1995).
Hernandez, L. et al. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 94, 3265–3268 (1999).
Schecterson, L. C. & Bothwell, M. Neurotrophin receptors: old friends with new partners. Dev. Neurobiol. 70, 332–338 (2010).
Klesse, L. J. & Parada, L. F. Trks: signal transduction and intracellular pathways. Microsc. Res. Tech. 45, 210–216 (1999).
Greco, A. et al. Role of the TFG N-terminus and coiled-coil domain in the transforming activity of the thyroid TRK-T3 oncogene. Oncogene 16, 809–816 (1998).
Zhang, F., Kartner, N. & Lukacs, G. L. Limited proteolysis as a probe for arrested conformational maturation of delta F508 CFTR. Nat. Struct. Biol. 5, 180–183 (1998).
Trzcinska-Daneluti, A. M. et al. High-content functional screen to identify proteins that correct F508del-CFTR function. Mol. Cell Proteomics. 8, 780–790 (2009).
Heinzer, S., Worz, S., Kalla, C., Rohr, K. & Weiss, M. A model for the self-organization of exit sites in the endoplasmic reticulum. J. Cell Sci. 121, 55–64 (2008).
Farhan, H., Weiss, M., Tani, K., Kaufman, R. J. & Hauri, H. P. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 27, 2043–2054 (2008).
Hughes, H. & Stephens, D. J. Assembly, organization, and function of the COPII coat. Histochem. Cell Biol. 129, 129–151 (2008).
Farhan, H. et al. MAPK signalling to the early secretory pathway revealed by kinase/phosphatase functional screening. J. Cell Biol. 189, 997–1011 (2010).
Cheeseman, I. M. et al. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 18, 2255–2268 (2004).
Audhya, A. et al. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J. Cell Biol. 171, 267–279 (2005).
Sato, K. et al. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol. Biol. Cell 17, 3085–3094 (2006).
Presley, J. F. et al. ER-to-Golgi transport visualized in living cells. Nature 389, 81–85 (1997).
Rostaing, P. et al. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24, 3463–3474 (2006).
Reynolds, E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–223 (1963).
Fiala, J. C. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005).
Perlaky, L. et al. Increased growth of NIH/3T3 cells by transfection with human p120 complementary DNA and inhibition by a p120 antisense construct. Cancer Res. 52, 428–436 (1992).
Saito, K. et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell 136, 891–902 (2009).
Diop, S. B. et al. Reptin and pontin function antagonistically with PcG and TrxG complexes to mediate Hox gene control. EMBO Rep. 9, 260–266 (2006).
Eng, J., McCormack, A. & Yates, J. R. III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 979–989 (1994).
Siegel, L. M. & Monty, K. J. Determination of molecular weights and frictional rations of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim. Biophys. Acta 112, 346–362 (1966).
Acknowledgements
This work was supported in part by grants from the NIH (1R01GM088151-01A1 to A.A. and P41RR011823 to J.R.Y.). We thank B. Weaver and P. Bertics for use of tissue culture facilities, R. Landick for use of a Gradient Master, K. Oegema for marker strains and antibodies, E. Chapman for use of Metamorph software and S. Koenig for help with RNAi. We also thank D. Stephens, P. Kiley and members of the Audhya laboratory for critically reading this manuscript.
Author information
Authors and Affiliations
Contributions
K.W., A.L.S., S.E. and A.A. conceived and designed experiments. K.W., A.L.S., A.S., J.H., J.R.M., K.S., S.E. and A.A. carried out experiments and analysed data. S.E., J.R.Y. and A.A. contributed reagents, materials and analysis tools. A.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1432 kb)
Supplementary Movie 1
Supplementary Information (WMV 1443 kb)
Supplementary Movie 2
Supplementary Information (MOV 3094 kb)
Rights and permissions
About this article
Cite this article
Witte, K., Schuh, A., Hegermann, J. et al. TFG-1 function in protein secretion and oncogenesis. Nat Cell Biol 13, 550–558 (2011). https://doi.org/10.1038/ncb2225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2225
This article is cited by
-
Nutrient deprivation alters the rate of COPII subunit recruitment at ER subdomains to tune secretory protein transport
Nature Communications (2023)
-
Clinical genetics of Charcot–Marie–Tooth disease
Journal of Human Genetics (2023)
-
Involvement of neuronal and muscular Trk-fused gene (TFG) defects in the development of neurodegenerative diseases
Scientific Reports (2022)
-
TRK-fused gene (TFG) regulates ULK1 stability via TRAF3-mediated ubiquitination and protects macrophages from LPS-induced pyroptosis
Cell Death & Disease (2022)
-
Homozygous TFG gene variants expanding the mutational and clinical spectrum of hereditary spastic paraplegia 57 and a review of literature
Journal of Human Genetics (2021)