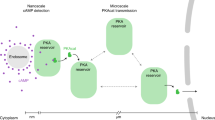
Abstract
Activation of G-protein-coupled receptors (GPCRs) mobilizes compartmentalized pulses of cyclic AMP. The main cellular effector of cAMP is protein kinase A (PKA), which is assembled as an inactive holoenzyme consisting of two regulatory (R) and two catalytic (PKAc) subunits. cAMP binding to R subunits dissociates the holoenzyme and releases the catalytic moiety, which phosphorylates a wide array of cellular proteins. Reassociation of PKAc and R components terminates the signal. Here we report that the RING ligase praja2 controls the stability of mammalian R subunits. Praja2 forms a stable complex with, and is phosphorylated by, PKA. Rising cAMP levels promote praja2-mediated ubiquitylation and subsequent proteolysis of compartmentalized R subunits, leading to sustained substrate phosphorylation by the activated kinase. Praja2 is required for efficient nuclear cAMP signalling and for PKA-mediated long-term memory. Thus, praja2 regulates the total concentration of R subunits, tuning the strength and duration of PKA signal output in response to cAMP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
22 March 2011
In the version of this article initially published online Figures 2, 4 and 5 were mislabelled, and on page 5 line 21 a sentence has now been reworded for clarity.
References
Taylor, S. S. et al. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim. Biophys. Acta 1784, 16–26 (2008).
Amieux, P. S. & McKnight, G. S. The essential role of RI α in the maintenance of regulated PKA activity. Ann. NY Acad. Sci. 968, 75–95 (2002).
Feliciello, A., Gottesman, M. E. & Avvedimento, E. V. The biological functions of A-kinase anchor proteins. J. Mol. Biol. 308, 99–114 (2001).
Tasken, K. & Aandahl, E. M. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 (2004).
Beene, D. L. & Scott, J. D. A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol. 19, 192–198 (2007).
Dell’Acqua, M. L. et al. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur. J. Cell Biol. 85, 627–633 (2006).
Dessauer, C. W. Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol. Pharmacol. 76, 935–941 (2009).
Zhong, H. et al. Subcellular dynamics of type II PKA in neurons. Neuron 62, 363–374 (2009).
Carlucci, A., Lignitto, L. & Feliciello, A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol. 18, 604–613 (2008).
Tunquist, B. J. et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc. Natl Acad. Sci. USA 105, 12557–12562 (2008).
Feliciello, A., Li, Y., Avvedimento, E. V., Gottesman, M. E. & Rubin, C. S. A-kinase anchor protein 75 increases the rate and magnitude of cAMP signaling to the nucleus. Curr. Biol. 7, 1011–1014 (1997).
Harada, H. et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3, 413–422 (1999).
Feliciello, A., Gottesman, M. E. & Avvedimento, E. V. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 17, 279–287 (2005).
Gomez, L. L., Alam, S., Smith, K. E., Horne, E. & Dell’Acqua, M. L. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J. Neurosci. 22, 7027–7044 (2002).
Houslay, M. D. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 35, 91–100 (2010).
Willoughby, D., Wong, W., Schaack, J., Scott, J. D. & Cooper, D. M. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J. 25, 2051–2061 (2006).
Smith, F. D. et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 12, 1242–1249 (2010).
Jurado, S., Biou, V. & Malenka, R. C. A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat. Neurosci. 13, 1053–1055 (2010).
Mauban, J. R., O’Donnell, M., Warrier, S., Manni, S. & Bond, M. AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology (Bethesda) 24, 78–87 (2009).
Bhattacharyya, S., Biou, V., Xu, W., Schluter, O. & Malenka, R. C. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 12, 172–181 (2009).
Lim, C. J. et al. α4 integrins are type I cAMP-dependent protein kinase-anchoring proteins. Nat. Cell Biol. 9, 415–421 (2007).
Carlucci, A. et al. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J. 27, 1073–1084 (2008).
Huang, Y. Y. & Kandel, E. R. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn. Mem. 1, 74–82 (1994).
Chain, D. G. et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron 22, 147–156 (1999).
Chain, D. G., Hegde, A. N., Yamamoto, N., Liu-Marsh, B. & Schwartz, J. H. Persistent activation of cAMP-dependent protein kinase by regulated proteolysis suggests a neuron-specific function of the ubiquitin system in Aplysia. J. Neurosci. 15, 7592–7603 (1995).
Chain, D. G., Schwartz, J. H. & Hegde, A. N. Ubiquitin-mediated proteolysis in learning and memory. Mol. Neurobiol. 20, 125–142 (1999).
Hegde, A. N. et al. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell 89, 115–126 (1997).
Feliciello, A. et al. The localization and activity of cAMP-dependent protein kinase affect cell cycle progression in thyroid cells. J. Biol. Chem. 275, 303–311 (2000).
Yu, P., Chen, Y., Tagle, D. A. & Cai, T. PJA1, encoding a RING-H2 finger ubiquitin ligase, is a novel human X chromosome gene abundantly expressed in brain. Genomics 79, 869–874 (2002).
Nakayama, M., Miyake, T., Gahara, Y., Ohara, O. & Kitamura, T. A novel RING-H2 motif protein downregulated by axotomy: its characteristic localization at the postsynaptic density of axosomatic synapse. J. Neurosci. 15, 5238–5248 (1995).
Rubino, H. M., Dammerman, M., Shafit-Zagardo, B. & Erlichman, J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron 3, 631–638 (1989).
Bregman, D. B., Hirsch, A. H. & Rubin, C. S. Molecular characterization of bovine brain P75, a high affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II β. J. Biol. Chem. 266, 7207–7213 (1991).
Glantz, S. B., Amat, J. A. & Rubin, C. S. cAMP signaling in neurons: patterns of neuronal expression and intracellular localization for a novel protein, AKAP 150, that anchors the regulatory subunit of cAMP-dependent protein kinase II β. Mol. Biol. Cell 3, 1215–1228 (1992).
Zhou, Y. et al. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J. Neurosci. 27, 13843–13853 (2007).
Michnick, S. W., Ear, P. H., Manderson, E. N., Remy, I. & Stefan, E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat. Rev. Drug Discov. 6, 569–582 (2007).
Stefan, E. et al. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc. Natl Acad. Sci. USA 104, 16916–16921 (2007).
Lavoie, C. et al. β1/β2-adrenergic receptor heterodimerization regulates β 2-adrenergic receptor internalization and ERK signaling efficacy. J. Biol. Chem. 277, 35402–35410 (2002).
Lonze, B. E. & Ginty, D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 (2002).
Montminy, M. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66, 807–822 (1997).
Malleret, G. et al. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J. Neurosci. 30, 3813–3825 (2010).
Armstrong, R., Wen, W., Meinkoth, J., Taylor, S. & Montminy, M. A refractory phase in cyclic AMP-responsive transcription requires down regulation of protein kinase A. Mol. Cell Biol. 15, 1826–1832 (1995).
Canettieri, G. et al. Attenuation of a phosphorylation-dependent activator by an HDAC–PP1 complex. Nat. Struct. Biol. 10, 175–181 (2003).
Houslay, M. D., Baillie, G. S. & Maurice, D. H. cAMP-specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ. Res. 100, 950–966 (2007).
Davis, G. W., DiAntonio, A., Petersen, S. A. & Goodman, C. S. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron 20, 305–315 (1998).
Amieux, P. S. et al. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J. Biol. Chem. 277, 27294–27304 (2002).
Newhall, K. J., Cummings, D. E., Nolan, M. A. & McKnight, G. S. Deletion of the RIIβ-subunit of protein kinase A decreases body weight and increases energy expenditure in the obese, leptin-deficient ob/ob mouse. Mol. Endocrinol. 19, 982–991 (2005).
Cummings, D. E. et al. Genetically lean mice result from targeted disruption of the RII β subunit of protein kinase A. Nature 382, 622–626 (1996).
Hegde, A. N., Goldberg, A. L. & Schwartz, J. H. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc. Natl Acad. Sci. USA 90, 7436–7440 (1993).
Acknowledgements
This work was supported by Grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and the Italian Ministry of University and Research (MIUR) 2008–2009 (2007KS47FW_002). E.S. was supported from Austrian Science Fund (FWF) Grant P22608 and Junior Researcher Support 2009 (University of Innsbruck). Special thanks to M. Gottesman, E. Avvedimento and K. Bister for discussion and critical reading of the manuscript, D. Viaggiano for help in immunohistochemistry and confocal microscopy analysis, R. D. Donne for help in carrying out experiments and S. McKnight and M. Ginsberg for providing the vectors for epitope-tagged R subunits. This manuscript is dedicated to the memory of M. Graziano.
Author information
Authors and Affiliations
Contributions
L.L., L.A., E.S. and A.F. designed the experiments. L.L. carried out most of the experiments except for the following:
Figs 2c, 7c,d and Supplementary Figs S5 and S7b were carried out by A.S., C.S. and O.C.
Figs 1d and 5g were carried out by M.S., who also generated praja2-deletion mutants.
Figs 3f–h, 5c–f, i, j, m and Supplementary Fig. S6b were carried out by E.S.
Figs 2a,b, 5a, 6a and Supplementary Figs S3 and S4 were carried out by C.G.
Figs 6g,h, 7e and Supplementary Fig. S7a were carried out by A.C.
Fig. 7f,g were carried out by R.N.
L.L., E.S., L.A. and A.F. analysed the data.
A.F. wrote the manuscript with contributions from L.A. and E.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 940 kb)
Rights and permissions
About this article
Cite this article
Lignitto, L., Carlucci, A., Sepe, M. et al. Control of PKA stability and signalling by the RING ligase praja2. Nat Cell Biol 13, 412–422 (2011). https://doi.org/10.1038/ncb2209
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2209
This article is cited by
-
Targeted inhibition of ubiquitin signaling reverses metabolic reprogramming and suppresses glioblastoma growth
Communications Biology (2022)
-
CD1d- and PJA2-related immune microenvironment differs between invasive breast carcinomas with and without a micropapillary feature
BMC Cancer (2019)
-
Feedback inhibition of cAMP effector signaling by a chaperone-assisted ubiquitin system
Nature Communications (2019)
-
Differential expression of the protein kinase A subunits in normal adrenal glands and adrenocortical adenomas
Scientific Reports (2017)
-
Ubiquitylation of MFHAS1 by the ubiquitin ligase praja2 promotes M1 macrophage polarization by activating JNK and p38 pathways
Cell Death & Disease (2017)