Abstract
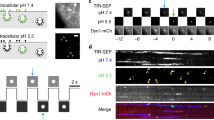
Clathrin-mediated endocytosis (CME) is the best-studied pathway by which cells selectively internalize molecules from the plasma membrane and surrounding environment. Previous live-cell imaging studies using ectopically overexpressed fluorescent fusions of endocytic proteins indicated that mammalian CME is a highly dynamic but inefficient and heterogeneous process. In contrast, studies of endocytosis in budding yeast using fluorescent protein fusions expressed at physiological levels from native genomic loci have revealed a process that is very regular and efficient. To analyse endocytic dynamics in mammalian cells in which endogenous protein stoichiometry is preserved, we targeted zinc finger nucleases (ZFNs) to the clathrin light chain A and dynamin-2 genomic loci and generated cell lines expressing fluorescent protein fusions from each locus. The genome-edited cells exhibited enhanced endocytic function, dynamics and efficiency when compared with previously studied cells, indicating that CME is highly sensitive to the levels of its protein components. Our study establishes that ZFN-mediated genome editing is a robust tool for expressing protein fusions at endogenous levels to faithfully report subcellular localization and dynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Merrifield, C. J., Feldman, M. E., Wan, L. & Almers, W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4, 691–698 (2002).
Pucadyil, T. J. & Schmid, S. L. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135, 1263–1275 (2008).
Roux, A., Uyhazi, K., Frost, A. & De Camilli, P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 (2006).
Goldstein, J. L. & Brown, M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 46, 897–930 (1977).
Zuchner, S. et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 37, 289–294 (2005).
Moradpour, D., Penin, F. & Rice, C. M. Replication of hepatitis C virus. Nat. Rev. Microbiol. 5, 453–463 (2007).
Brodsky, F. M., Chen, C. Y., Knuehl, C., Towler, M. C. & Wakeham, D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568 (2001).
Kaksonen, M., Toret, C. P. & Drubin, D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305–320 (2005).
Rappoport, J. Z., Kemal, S., Benmerah, A. & Simon, S. M. Dynamics of clathrin and adaptor proteins during endocytosis. Am. J. Physiol. 291, C1072–C1081 (2006).
Saffarian, S., Cocucci, E. & Kirchhausen, T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. Plos Biol. 7, e1000191 (2009).
Loerke, D. et al. Cargo and dynamin regulate clathrin-coated pit maturation. Plos Biol. 7, 628–639 (2009).
Ehrlich, M. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Merrifield, C. J., Qualmann, B., Kessels, M. M. & Almers, W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur. J. Cell Biol. 83, 13–18 (2004).
Soulet, F., Yarar, D., Leonard, M. & Schmid, S. L. SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol. Biol. Cell 16, 2058–2067 (2005).
Knoops, L., Hornakova, T., Royer, Y., Constantinescu, S. N. & Renauld, J. C. JAK kinases overexpression promotes in vitro cell transformation. Oncogene 27, 1511–1519 (2008).
Kuma, A., Matsui, M. & Mizushima, N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy 3, 323–328 (2007).
Luo, T., Matsuo-Takasaki, M. & Sargent, T. D. Distinct roles for Distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Mol. Reprod. Dev. 60, 331–337 (2001).
Miyama, K. et al. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev. Biol. 208, 123–133 (1999).
Ward, C. L., Omura, S. & Kopito, R. R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 (1995).
Jensen, T. J. et al. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135 (1995).
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646 (2010).
Mettlen, M., Loerke, D., Yarar, D., Danuser, G. & Schmid, S. L. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J. Cell Biol. 188, 919–933 (2010).
Mettlen, M. et al. Endocytic accessory proteins are functionally distinguished by their differential effects on the maturation of clathrin-coated pits. Mol. Biol. Cell 20, 3251–3260 (2009).
Liu, Y. W., Surka, M. C., Schroeter, T., Lukiyanchuk, V. & Schmid, S. L. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol. Biol. Cell 19, 5347–5359 (2008).
Huang, F., Khvorova, A., Marshall, W. & Sorking, A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol. Chem. 279, 16657–16661 (2004).
Mettlen, M., Pucadyil, T., Ramachandran, R. & Schmid, S. L. Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem. Soc. Trans. 37, 1022–1026 (2009).
Kirchhausen, T. Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 19, 596–605 (2009).
Le Clainche, C. et al. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 26, 1199–1210 (2007).
Merrifield, C. J., Perrais, D. & Zenisek, D. Coupling between clathrin-coated-pit invagination, cortactin recruitment and membrane scission observed in live cells. Cell 121, 593–606 (2005).
DeKelver, R. C. et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 20, 1133–1142 (2010).
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857 (2009).
Isalan, M., Klug, A. & Choo, Y. A rapid, generally applicable method to engineer zinc fingers illustrated by targeting the HIV-1 promotor. Nat. Biotechnol. 19, 656–660 (2001).
Urnov, F. D. et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651 (2005).
DeKelver, R. C. et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 20, 1133–1142 (2010).
Doyon, Y. et al. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods 8, 74–79 (2011).
Wu, X. et al. Clathrin exchange during clathrin-mediated endocytosis. J. Cell Biol. 155, 291–300 (2001).
Shaner, N. C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5, 545–551 (2008).
Doyon, Y. et al. Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat. Methods 7, 459–460 (2010).
Acknowledgements
We thank H. Nolla and A. Valeros for their cell-sorting expertise, A. Fischer and M. Yasukawa for help with cell culture, the Biological Imaging Facility for use of the Imaris software, the Sangamo Production group for technical assistance and members of the Drubin/Barnes lab for critical reading of this manuscript. We also thank T. Kirchhausen, L. Greene, and R. Tsien for providing the BSC-1 GFP–CLTA cell line, human CLTA plasmid, and TagRFP-T plasmid, respectively. J.B.D. and J.C. were supported by postdoctoral fellowships from the Jane Coffin Childs Memorial Fund and The Croucher Foundation, respectively. This work was supported by NIH grant R01 GM65462 to D.G.D.
Author information
Authors and Affiliations
Contributions
J.B.D, B.Z., J.C., A.T.C., T.D.V., Y.D., J.C.M., D.E.P., L.Z., E.J.R., P.D.G., F.D.U. and D.G.D designed the study and experiments. J.B.D, B.Z., J.C., A.T.C., J.M.C., Y.S. and A.H.L. performed the experiments. J.B.D., B.Z., J.C., A.T.C., J.M.C. and F.D.U. analysed the data. J.B.D., B.Z., J.C., A.T.C., F.D.U. and D.G.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.Z., J.M.C., Y.S., A.H.L., T.D.V., Y.D., J.C.M., D.E.P., L.Z., E.J.R., P.D.G. and F.D.U are full-time employees of Sangamo BioSciences, Incorporated.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2200 kb)
Supplementary Information Movie S1
Supplementary Information (MOV 1990 kb)
Supplementary Information Movie S2
Supplementary Information (MOV 1862 kb)
Supplementary Information Movie S3
Supplementary Information (MOV 2352 kb)
Supplementary Information Movie S4
Supplementary Information (MOV 2465 kb)
Supplementary Information Movie S5
Supplementary Information (MOV 1933 kb)
Supplementary Information Movie S6
Supplementary Information (MOV 1736 kb)
Supplementary Information Movie S7
Supplementary Information (MOV 2573 kb)
Supplementary Information Movie S8
Supplementary Information (MOV 1745 kb)
Supplementary Information Movie S9
Supplementary Information (MOV 2063 kb)
Rights and permissions
About this article
Cite this article
Doyon, J., Zeitler, B., Cheng, J. et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat Cell Biol 13, 331–337 (2011). https://doi.org/10.1038/ncb2175
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2175
This article is cited by
-
A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells
Nature Communications (2023)
-
Branched actin networks are organized for asymmetric force production during clathrin-mediated endocytosis in mammalian cells
Nature Communications (2022)
-
Actin polymerization promotes invagination of flat clathrin-coated lattices in mammalian cells by pushing at lattice edges
Nature Communications (2022)
-
Imaging vesicle formation dynamics supports the flexible model of clathrin-mediated endocytosis
Nature Communications (2022)
-
The EMT activator ZEB1 accelerates endosomal trafficking to establish a polarity axis in lung adenocarcinoma cells
Nature Communications (2021)