Abstract
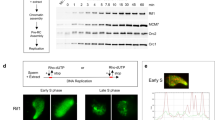
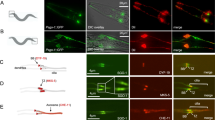
Many of the factors required for chromosomal DNA replication have been identified in unicellular eukaryotes. However, DNA replication is poorly understood in multicellular organisms. Here, we report the identification of GEMC1 (geminin coiled-coil containing protein 1), a novel vertebrate protein required for chromosomal DNA replication. GEMC1 is highly conserved in vertebrates and is preferentially expressed in proliferating cells. Using Xenopus laevis egg extract we show that Xenopus GEMC1 (xGEMC1) binds to the checkpoint and replication factor TopBP1, which promotes binding of xGEMC1 to chromatin during pre-replication complex (pre-RC) formation. We demonstrate that xGEMC1 interacts directly with replication factors such as Cdc45 and the kinase Cdk2–CyclinE, through which it is heavily phosphorylated. Phosphorylated xGEMC1 stimulates initiation of DNA replication, whereas depletion of xGEMC1 prevents the onset of DNA replication owing to the impairment of Cdc45 loading onto chromatin. Similarly, inhibition of GEMC1 expression with morpholino and siRNA oligos prevents DNA replication in embryonic and somatic vertebrate cells. These data suggest that GEMC1 promotes initiation of chromosomal DNA replication in multicellular organisms by mediating TopBP1- and Cdk2-dependent recruitment of Cdc45 onto replication origins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Diffley, J. F. Regulation of early events in chromosome replication. Curr. Biol. 14, R778–786 (2004).
Takisawa, H., Mimura, S. & Kubota, Y. Eukaryotic DNA replication: from pre-replication complex to initiation complex. Curr. Opin. Cell. Biol. 12, 690–696 (2000).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Saxena, S. et al. A dimerized coiled-coil domain and an adjoining part of geminin interact with two sites on Cdt1 for replication inhibition. Mol. Cell 15, 245–258 (2004).
Costanzo, V. & Gautier, J. Xenopus cell-free extracts to study DNA damage checkpoints. Methods Mol. Biol. 241, 255–267 (2004).
Trenz, K., Errico, A. & Costanzo, V. Plx1 is required for chromosomal DNA replication under stressful conditions. EMBO J. 27, 876–885 (2008).
Hashimoto, Y. & Takisawa, H. Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J. 22, 2526–2535 (2003).
Newport, J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell 48, 205–217 (1987).
Dunphy, W. G., Brizuela, L., Beach, D. & Newport, J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54, 423–431 (1988).
Luciani, M. G., Oehlmann, M. & Blow, J. J. Characterization of a novel ATR-dependent, Chk1-independent, intra-S phase checkpoint that suppresses initiation of replication in Xenopus. J. Cell Sci. 117, 6019–6030 (2004).
Mimura, S., Seki, T., Tanaka, S. & Diffley, J. F. Phosphorylation-dependent binding of mitotic cyclins to Cdc6 contributes to DNA replication control. Nature 431, 1118–1123 (2004).
Schulman, B. A., Lindstrom, D. L. & Harlow, E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin, A. Proc. Natl. Acad. Sci. USA 95, 10453–10458 (1998).
Blow, J. J. & Laskey, R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47, 577–587 (1986).
Krasinska, L. et al. Cdk1 and Cdk2 activity levels determine the efficiency of replication origin firing in Xenopus. EMBO J. 27, 758–769 (2008).
Shechter, D., Costanzo, V. & Gautier, J. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell. Biol. 6, 648–655 (2004).
Khokha, M. K. et al. Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499–510 (2002).
Kubota, Y. et al. A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev. 17, 1141–1152 (2003).
Van Hatten, R. A. et al. The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J. Cell Biol. 159, 541–547 (2002).
Wohlschlegel, J. A., Dhar, S. K., Prokhorova, T. A., Dutta, A. & Walter, J. C. Xenopus Mcm10 binds to origins of DNA replication after Mcm2–7 and stimulates origin binding of Cdc45. Mol. Cell 9, 233–240 (2002).
Walter, J. & Newport, J. Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase α. Mol. Cell 5, 617–627 (2000).
Mimura, S. & Takisawa, H. Xenopus Cdc45-dependent loading of DNA polymerase α onto chromatin under the control of S phase Cdk. EMBO J. 17, 5699–5707 (1998).
Tanaka, T. & Nasmyth, K. Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases. EMBO J. 17, 5182–5191 (1998).
Zegerman, P. & Diffley, J. F. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445, 281–285 (2007).
Tanaka, S. et al. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445, 328–332 (2007).
Kamimura, Y., Tak, Y. S., Sugino, A. & Araki, H. Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J. 20, 2097–2107 (2001).
Nakajima, R. & Masukata, H. SpSld3 is required for loading and maintenance of SpCdc45 on chromatin in DNA replication in fission yeast. Mol. Biol. Cell 13, 1462–1472 (2002).
Tanaka, S., Tak, Y. S. & Araki, H. The role of CDK in the initiation step of DNA replication in eukaryotes. Cell Div. 2, 16 (2007).
Sangrithi, M. N. et al. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell 121, 887–898 (2005).
Matsuno, K., Kumano, M., Kubota, Y., Hashimoto, Y. & Takisawa, H. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase α in the initiation of DNA replication. Mol. Cell Biol. 26, 4843–4852 (2006).
Kubota, Y. & Takisawa, H. Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J. Cell Biol. 123, 1321–1331 (1993).
Hensey, C. & Gautier, J. A developmental timer that regulates apoptosis at the onset of gastrulation. Mech. Dev. 69, 183–195 (1997).
Slack, J. Embryonic development. Cell communication in early embryos. Nature 311, 107–108 (1984).
Khokha, M. K. et al. Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499–510 (2002).
Li, A. & Blow, J. J. Cdt1 downregulation by proteolysis and geminin inhibition prevents DNA re-replication in Xenopus. EMBO J. 24, 395–404 (2005).
Marheineke, K., Goldar, A., Krude, T. & Hyrien, O. Use of DNA combing to study DNA replication in Xenopus and human cell-free systems. Methods Mol. Biol. 521, 575–603 (2009).
Chong, J. P., Thommes, P., Rowles, A., Mahbubani, H. M. & Blow, J. J. Characterization of the Xenopus replication licensing system. Methods Enzymol. 283, 549–564 (1997).
Mechali, M. & Harland, R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell 30, 93–101 (1982).
Acknowledgements
We thank T. Hunt, members of Clare Hall Laboratories and members of the Genome Stability Unit for their comments, H. Mahbubani and J. Kirk for technical support with X. laevis, J. Gannon for technical assistance in antibody production and M. Wu for embryo injection. This work was supported by grants from Cancer Research UK. V. C. is also supported by the Lister Institute of Preventive Medicine, the European Research Council (ERC) start-up grant (N. 206281) and the EMBO Young Investigator Program (YIP).
Author information
Authors and Affiliations
Contributions
A.B, C.C, A.E and E.G performed the experiments. V.C conceived the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1038 kb)
Rights and permissions
About this article
Cite this article
Balestrini, A., Cosentino, C., Errico, A. et al. GEMC1 is a TopBP1-interacting protein required for chromosomal DNA replication. Nat Cell Biol 12, 484–491 (2010). https://doi.org/10.1038/ncb2050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2050
This article is cited by
-
The genetic architecture of fornix white matter microstructure and their involvement in neuropsychiatric disorders
Translational Psychiatry (2023)
-
GEMC1 and MCIDAS interactions with SWI/SNF complexes regulate the multiciliated cell-specific transcriptional program
Cell Death & Disease (2023)
-
Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer’s disease
Molecular Neurodegeneration (2022)
-
Molecular causes of primary microcephaly and related diseases: a report from the UNIA Workshop
Chromosoma (2020)
-
Multicilin and activated E2f4 induce multiciliated cell differentiation in primary fibroblasts
Scientific Reports (2018)