Abstract
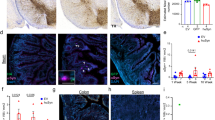
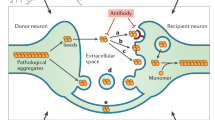
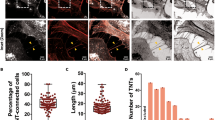
In variant Creutzfeldt–Jakob disease, prions (PrPSc) enter the body with contaminated foodstuffs and can spread from the intestinal entry site to the central nervous system (CNS) by intercellular transfer from the lymphoid system to the peripheral nervous system (PNS)1. Although several means2,3,4 and different cell types5,6,7 have been proposed to have a role, the mechanism of cell-to-cell spreading remains elusive. Tunnelling nanotubes (TNTs) have been identified between cells8,9,10,11,12, both in vitro and in vivo10,11,13, and may represent a conserved means of cell-to-cell communication14,15,16. Here we show that TNTs allow transfer of exogenous and endogenous PrPSc between infected and naive neuronal CAD cells17. Significantly, transfer of endogenous PrPSc aggregates was detected exclusively when cells chronically infected with the 139A mouse prion strain were connected to mouse CAD cells by means of TNTs, identifying TNTs as an efficient route for PrPSc spreading in neuronal cells. In addition, we detected the transfer of labelled PrPSc from bone marrow-derived dendritic cells to primary neurons connected through TNTs. Because dendritic cells can interact with peripheral neurons in lymphoid organs, TNT-mediated intercellular transfer would allow neurons to transport prions retrogradely to the CNS1. We therefore propose that TNTs are involved in the spreading of PrPSc within neurons in the CNS and from the peripheral site of entry to the PNS by neuroimmune interactions with dendritic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mabbott, N. A. & MacPherson, G. G. Prions and their lethal journey to the brain. Nature Rev. Microbiol. 4, 201–11 (2006).
Fevrier, B. et al. Cells release prions in association with exosomes. Proc. Natl Acad. Sci. USA 101, 9683–9688 (2004).
Leblanc, P. et al. Retrovirus infection strongly enhances scrapie infectivity release in cell culture. EMBO J. 25, 2674–2685 (2006).
Liu, T. et al. Intercellular transfer of the cellular prion protein. J. Biol. Chem. 277, 47671–47678 (2002).
Aucouturier, P. et al. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Invest. 108, 703–708 (2001).
Montrasio, F. et al. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288, 1257–1259 (2000).
Prinz, M. et al. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc. Natl Acad. Sci. USA 99, 919–924 (2002).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H. H. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004).
Onfelt, B., Nedvetzki, S., Yanagi, K. & Davis, D. M. Cutting edge: Membrane nanotubes connect immune cells. J. Immunol. 173, 1511–1513 (2004).
Hsiung, F., Ramirez-Weber, F. A., Iwaki, D. D. & Kornberg, T. B. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437, 560–563 (2005).
Ramirez-Weber, F. A. & Kornberg, T. B. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607 (1999).
Sherer, N. M. et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biol. 9, 310–315 (2007).
Chinnery, H. R., Pearlman, E. & McMenamin, P. G. Cutting edge: Membrane nanotubes in vivo: a feature of MHCII+ cells in the mouse cornea. J. Immunol. 180, 5779–5783 (2008).
Demontis, F. & Dahmann, C. Apical and lateral cell protrusions interconnect epithelial cells in live Drosophila wing imaginal discs. Dev. Dyn. 236, 3408–3418 (2007).
Gerdes, H.-H., Bukoreshtliev, N. V. & Barroso, J. F. V. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 581, 2194–2201 (2007).
Sowinski, S. et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature Cell Biol. 10, 212–219 (2008).
Qi, Y., Wang, J. K. T., McMillian, M. & Chikaraishi, D. M. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 17, 1217–1225 (1997).
Mahal, S. P. et al. Prion strain discrimination in cell culture: the cell panel assay. Proc. Natl Acad. Sci. USA 104, 20908–20913 (2007).
Sherer, N. M. & Mothes, W. Cytonemes and tunneling nanotubules in cell–cell communication and viral pathogenesis. Trends Cell Biol. 18, 414–420 (2008).
Reed, B. C. et al. GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol. Biol. Cell 16, 4183–4201 (2005).
Howard, J. Molecular motors: structural adaptations to cellular functions. Nature 389, 561–567 (1997).
Magalhaes, A. C. et al. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 25, 5207–5216 (2005).
Taraboulos, A., Serban, D. & Prusiner, S. B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110, 2117–2132 (1990).
Watkins, S. C. & Salter, R. D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23, 309–318 (2005).
Huang, F. P., Farquhar, C. F., Mabbott, N. A., Bruce, M. E. & MacPherson, G. G. Migrating intestinal dendritic cells transport PrPSc from the gut. J. Gen. Virol. 83, 267–271 (2002).
McBride, P. A. & Beekes, M. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci. Lett. 265, 135–138 (1999).
Lambrecht, B. N. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir. Res. 2, 133–138 (2001).
Defaweux, V. et al. Interfaces between dendritic cells, other immune cells, and nerve fibres in mouse Peyer's patches: potential sites for neuroinvasion in prion diseases. Microsc. Res. Tech. 66, 1–9 (2005).
Dorban, G. et al. Oral scrapie infection modifies the homeostasis of Peyer's patches' dendritic cells. Histochem. Cell Biol. 128, 243–251 (2007).
Luhr, K. et al. Scrapie protein degradation by cysteine proteases in CD11c+ dendritic cells and GT1-1 neuronal cells. J. Virol. 78, 4776–4782 (2004).
Vella, L. J. et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 211, 582–590 (2007).
Kanu, N. et al. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 12, 523–530 (2002).
Paquet, S. et al. Efficient dissemination of prions through preferential transmission to nearby cells. J. Gen. Virol. 88, 706–713 (2007).
Onfelt, B. et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177, 8476–8483 (2006).
Brejot, T. et al. Forced expression of the motor neuron determinant HB9 in neural stem cells affects neurogenesis. Exp. Neurol. 198, 167–182 (2006).
Cronier, S., Laude, H. & Peyrin, J. M. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl Acad. Sci. USA 101, 12271–12276 (2004).
Mederle, I. et al. Plasmidic versus insertional cloning of heterologous genes in Mycobacterium bovis BCG: impact on in vivo antigen persistence and immune responses. Infect. Immun. 70, 303–314 (2002).
Kaech, S. & Banker, G. Culturing hippocampal neurons. Nature Protocols 1, 2406–2415 (2006).
Acknowledgements
We thank P. Lazarow, G. Guizzunti and C. Bowler for critical reading of the manuscript. We thank S. Blanchard and D. Bohl-Delfaud for their help in preparing the GFP–PrPwt-retroviral vector, and P. Casanova and J. Vinatier for technical help. We thank H. Laude, A. F. Hill, P. Cossart and M. Way for their gifts (cells, constructs and reagents). We are grateful for assistance with microscopes and image processing received from the Plate-Forme Imagerie Dynamique at the Pasteur Institut. K.G. is supported by the Pasteur Foundation Fellowship Program, E.S. received a fellowship (2004-07) from the Bavarian Research Foundation (BFS), D.B. received funding from the Fondation Canadienne Louis Pasteur, and Z.M. received funding from Ile-de-France. This work was supported by grants to C.Z. from the European Union (Strainbarrier (FP6 Contract No 023183 (Food)) and from Telethon GGP0414.
Author information
Authors and Affiliations
Contributions
C.Z. and E.S. conceived the project. K.G. and E.S. planned and performed most of the experiments with TNTs in different cells and analysed the data. C.L. planned and performed the infection experiments and analysed the data. Z.M. and A.C. planned and performed experiments in fixed CAD cells and analysed the data. D.T.B. prepared the Alexa-PrPSc and discussed the experiments. N.C. and F.C. performed most of the quantitative image analysis under the supervision of J.C.O. J.E. helped with image reconstruction and discussed the data. A.M. prepared the BMDCs and discussed the related experiments. D.M. co-directed the PhD thesis of E.S. and discussed data with E.S. and C.Z. C.Z. coordinated the project and assisted with planning the experiments and data analysis. K.G., E.S. and C.Z. wrote the manuscript. All authors discussed the results and manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1520 kb)
Supplementary Information
Supplementary Movie 1 (MOV 3247 kb)
Supplementary Information
Supplementary Movie 2 (MOV 3390 kb)
Supplementary Information
Supplementary Movie 3 (MOV 5187 kb)
Supplementary Information
Supplementary Movie 4 (MOV 108 kb)
Supplementary Information
Supplementary Movie 5 (MOV 755 kb)
Supplementary Information
Supplementary Movie 6 (MOV 750 kb)
Supplementary Information
Supplementary Movie 7 (MOV 5175 kb)
Supplementary Information
Supplementary Movie 8 (MOV 518 kb)
Supplementary Information
Supplementary Movie 9 (MOV 4558 kb)
Supplementary Information
Supplementary Movie 10 (MOV 5476 kb)
Supplementary Information
Supplementary Movie 11 (MOV 4329 kb)
Supplementary Information
Supplementary Movie 12 (MOV 724 kb)
Supplementary Information
Supplementary Movie 13 (MOV 755 kb)
Rights and permissions
About this article
Cite this article
Gousset, K., Schiff, E., Langevin, C. et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11, 328–336 (2009). https://doi.org/10.1038/ncb1841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1841
This article is cited by
-
Brain clearance of protein aggregates: a close-up on astrocytes
Molecular Neurodegeneration (2024)
-
Rescue of mitochondrial import failure by intercellular organellar transfer
Nature Communications (2024)
-
Mitochondrial transfer in hematological malignancies
Biomarker Research (2023)
-
α-Synuclein conformers reveal link to clinical heterogeneity of α-synucleinopathies
Translational Neurodegeneration (2023)
-
Roles of neuropathology-associated reactive astrocytes: a systematic review
Acta Neuropathologica Communications (2023)