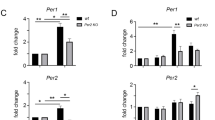
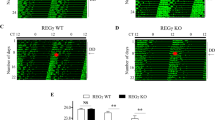
Abstract
The circadian clock is reset by external time cues for synchronization to environmental changes1. In mammals, the light-input signalling pathway mediated by Per gene induction has been extensively studied2,3. On the other hand, little is known about resetting mechanisms that are independent of Per induction4,5,6. Here we show that activation of activin receptor-like kinase (ALK), triggered by TGF-β, activin or alkali signals, evoked resetting of the cellular clock independently of Per induction. The resetting was mediated by an immediate-early induction of Dec1, a gene whose physiological role in the function of the circadian clock has been unclear. Acute Dec1 induction was a prerequisite for ALK-mediated resetting and upregulation was dependent on SMAD3, which was phosphorylated for activation in response to the resetting stimuli. Intraperitoneal injection of TGF-β into wild-type or Dec1-deficient mice demonstrated that Dec1 has an essential role in phase-shift of clock gene expression in the kidney and adrenal gland. These results indicate that ALK–SMAD3–Dec1 signalling provides an input pathway in the mammalian molecular clock.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Johnson, C.H., Elliott, J.A. & Foster, R. Entrainment of circadian programs. Chronobiol. Int. 20, 741–774 (2003).
Reppert, S.M. & Weaver, D.R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Hirota, T. & Fukada, Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zool. Sci. 21, 359–368 (2004).
Yannielli, P. & Harrington, M.E. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog. Neurobiol. 74, 59–76 (2004).
Hirota, T. et al. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured rat-1 fibroblasts. J. Biol. Chem. 277, 44244–44251 (2002).
Nakahata, Y., Akashi, M., Trcka, D., Yasuda, A. & Takumi, T. The in vitro real-time oscillation monitoring system identifies potential entrainment factors for circadian clocks. BMC Mol. Biol. 7, 5 (2006).
Schibler, U. & Sassone-Corsi, P. A web of circadian pacemakers. Cell 111, 919–922 (2002).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Pando, M.P., Morse, D., Cermakian, N. & Sassone-Corsi, P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell 110, 107–117 (2002).
Feillet, C.A. et al. Lack of food anticipation in Per2 mutant mice. Curr. Biol. 16, 2016–2022 (2006).
Balsalobre, A., Marcacci, L. & Schibler, U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 10, 1291–1294 (2000).
Akashi, M. & Nishida, E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev. 14, 645–649 (2000).
Crosthwaite, S.K., Loros, J.J. & Dunlap, J.C. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell 81, 1003–1012 (1995).
Shigeyoshi, Y. et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91, 1043–1053 (1997).
Inman, G.J. et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65–74 (2002).
Rena, G., Bain, J., Elliott, M. & Cohen, P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 5, 60–65 (2004).
Derynck, R. & Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Massague, J., Seoane, J. & Wotton, D. Smad transcription factors. Genes Dev. 19, 2783–2810 (2005).
Zawel, L. et al. DEC1 is a downstream target of TGF-β with sequence-specific transcriptional repressor activities. Proc. Natl Acad. Sci. USA 99, 2848–2853 (2002).
Ehata, S. et al. Transforming growth factor-β promotes survival of mammary carcinoma cells through induction of antiapoptotic transcription factor DEC1. Cancer Res. 67, 9694–9703 (2007).
Foster, C.M., Olton, P.R. & Padmanabhan, V. Diurnal changes in FSH-regulatory peptides and their relationship to gonadotrophins in pubertal girls. Hum. Reprod. 20, 543–548 (2005).
Inoue, K. et al. Transforming growth factor-β activated during exercise in brain depresses spontaneous motor activity of animals. Relevance To central fatigue. Brain Res. 846, 145–153 (1999).
Bennett, A.L. et al. Elevation of bioactive transforming growth factor-β in serum from patients with chronic fatigue syndrome. J. Clin. Immunol. 17, 160–166 (1997).
Tryon, W.W., Jason, L., Frankenberry, E. & Torres-Harding, S. Chronic fatigue syndrome impairs circadian rhythm of activity level. Physiol. Behav. 82, 849–853 (2004).
Khalil, N. TGF-β: from latent to active. Microbes Infect. 1, 1255–1263 (1999).
Dmitriev, A.V. & Mangel, S.C. Circadian clock regulation of pH in the rabbit retina. J. Neurosci. 21, 2897–2902 (2001).
Challet, E., Malan, A., Turek, F.W. & Van Reeth, O. Daily variations of blood glucose, acid-base state and PCO2 in rats: effect of light exposure. Neurosci. Lett. 355, 131–135 (2004).
Yamamoto, F., Borgula, G.A. & Steinberg, R.H. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp. Eye Res. 54, 685–697 (1992).
Honma, S. et al. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419, 841–844 (2002).
Kawamoto, T. et al. A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem. Biophys. Res. Commun. 313, 117–124 (2004).
Grechez-Cassiau, A. et al. The transcriptional repressor STRA13 regulates a subset of peripheral circadian outputs. J. Biol. Chem. 279, 1141–1150 (2004).
Nakashima, A et al. DEC1 modulates circadian phase of clock gene expression. Mol. Cell Biol. 28, 4080–4092 (2008).
Yamada, K. & Miyamoto, K. Basic helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their gene expressions are regulated by multiple extracellular stimuli. Front. Biosci. 10, 3151–71 (2005).
Preitner, N. et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Ueda, H.R. et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genet. 37, 187–192 (2005).
Acknowledgements
We thank T. Okano for valuable discussion; K. Sanada and H. Yoshitane for comments on the manuscripts; M. Akiyama for providing pGL3-Basic-Hyg reporter vector; K. Ui-Tei and Y. Naito for technical advice on RNA interference; M. Takekawa for technical advice for experiment with kinase inhibitors. This work was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology, by the 21st Century COE Program (Promotion of basic biosciences for the understanding of organisms' uniqueness), and by Global COE Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms) from MEXT, Japan.
Author information
Authors and Affiliations
Contributions
N. K. and T. H. developed the real-time monitoring assay system; N. K. performed the cell biological and animal experiments with assistance from T. H and Y. F.; T. K. and Y. K. generated Dec1−/− mice; T. T. quantified TGF-β by ELISA with assistance from N. K and Y. F; N. K., T. H. and Y. F. planned the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1680 kb)
Rights and permissions
About this article
Cite this article
Kon, N., Hirota, T., Kawamoto, T. et al. Activation of TGF-β/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol 10, 1463–1469 (2008). https://doi.org/10.1038/ncb1806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1806
This article is cited by
-
Singularity response reveals entrainment properties in mammalian circadian clock
Nature Communications (2023)
-
Real time monitoring of cold Ca2+ dependent transcription and its modulation by NCX inhibitors
Scientific Reports (2022)
-
Modulation of circadian clock by crude drug extracts used in Japanese Kampo medicine
Scientific Reports (2021)
-
Circa-SCOPE: high-throughput live single-cell imaging method for analysis of circadian clock resetting
Nature Communications (2021)
-
The role of clockwork orange in the circadian clock of the cricket Gryllus bimaculatus
Zoological Letters (2020)