Abstract
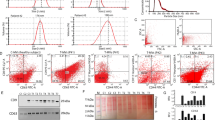
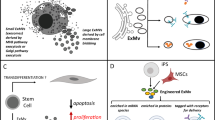
Glioblastoma tumour cells release microvesicles (exosomes) containing mRNA, miRNA and angiogenic proteins. These microvesicles are taken up by normal host cells, such as brain microvascular endothelial cells. By incorporating an mRNA for a reporter protein into these microvesicles, we demonstrate that messages delivered by microvesicles are translated by recipient cells. These microvesicles are also enriched in angiogenic proteins and stimulate tubule formation by endothelial cells. Tumour-derived microvesicles therefore serve as a means of delivering genetic information and proteins to recipient cells in the tumour environment. Glioblastoma microvesicles also stimulated proliferation of a human glioma cell line, indicating a self-promoting aspect. Messenger RNA mutant/variants and miRNAs characteristic of gliomas could be detected in serum microvesicles of glioblastoma patients. The tumour-specific EGFRvIII was detected in serum microvesicles from 7 out of 25 glioblastoma patients. Thus, tumour-derived microvesicles may provide diagnostic information and aid in therapeutic decisions for cancer patients through a blood test.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Stupp, R., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 352, 987–996 (2005).
Mazzocca, A., et al. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 65, 4728–4738 (2005).
Muerkoster, S., et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1β. Cancer Res. 64, 1331–1337 (2004).
Singer, C. F., et al. Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Res. Treat. 110, 273–281 (2008).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Gabrilovich, D. I. Molecular mechanisms and therapeutic reversal of immune suppression in cancer. Curr. Cancer Drug Targets 7, 1 (2007).
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A. & Ratajczak, M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495 (2006).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nature Rev. Immunol. 2, 569–579 (2002).
Pan, B. T. & Johnstone, R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 (1983).
Booth, A. M., et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 172, 923–935 (2006).
Greco, V., Hannus, M. & Eaton, S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106, 633–645 (2001).
Delves, G. H., Stewart, A. B., Cooper, A. J. & Lwaleed, B. A. Prostasomes, angiogenesis, and tissue factor. Semin. Thromb. Hemost. 33, 75–79 (2007).
Mack, M., et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nature Med. 6, 769–775 (2000).
Al-Nedawi, K., et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature Cell Biol. 10, 619–624 (2008).
Valadi, H., et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 9, 654–659 (2007).
Baj-Krzyworzeka, M., et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 55, 808–818 (2006).
Chaput, N., Taieb, J., Andre, F. & Zitvogel, L. The potential of exosomes in immunotherapy. Exp. Opin. Biol. Ther. 5, 737–747 (2005).
Wieckowski, E. & Whiteside, T. L. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol. Res. 36, 247–254 (2006).
Clayton, A., Mitchell, J. P., Court, J., Mason, M. D. & Tabi, Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 67, 7458–7466 (2007).
Ginestra, A., et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 18, 3433–3437 (1998).
Liu, C., et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176, 1375–1385 (2006).
Janowska-Wieczorek, A., et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 113, 752–760 (2005).
Millimaggi, D., et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 9, 349–357 (2007).
Tannous, B. A., Kim, D. E., Fernandez, J. L., Weissleder, R. & Breakefield, X. O. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 11, 435–443 (2005).
Pelloski, C. E., et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 25, 2288–2294 (2007).
Nishikawa, R., et al. Immunohistochemical analysis of the mutant epidermal growth factor, δEGFR, in glioblastoma. Brain Tumor Pathol. 21, 53–56 (2004).
Chan, J. A., Krichevsky, A. M. & Kosik, K. S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 65, 6029–6033 (2005).
Brat, D. J., Bellail, A. C. & Van Meir, E. G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncology 7, 122–133 (2005).
Eberle, K., et al. The expression of angiogenin in tissue samples of different brain tumours and cultured glioma cells. Anticancer Res. 20, 1679–1684 (2000).
Rolhion, C., et al. Interleukin-6 overexpression as a marker of malignancy in human gliomas. J. Neurosurg. 94, 97–101 (2001).
Taraboletti, G., et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia 8, 96–103 (2006).
Xu, Z. P., Tsuji, T., Riordan, J. F. & Hu, G. F. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry 42, 121–128 (2003).
Kislauskis, E. H., Zhu, X. & Singer, R. H. Sequences responsible for intracellular localization of β-actin messenger RNA also affect cell phenotype. J. Cell Biol. 127, 441–451 (1994).
Mallardo, M., et al. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc. Natl Acad. Sci. USA 100, 2100–2105 (2003).
Sonabend, A. M., Dana, K. & Lesniak, M. S. Targeting epidermal growth factor receptor variant III: a novel strategy for the therapy of malignant glioma. Exp. Rev. Anticancer Ther. 7, S45–S50 (2007).
Mellinghoff, I. K., et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. New Engl. J. Med. 353, 2012–2024 (2005).
Badr, C. E., Hewett, J. W., Breakefield, X. O. & Tannous, B. A. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS ONE 2, e571 (2007).
Acknowledgements
We wish to express our gratitude to B. Tannous for supplying the Gluc lentivirus construct, C. Maguire, M. Broekman, K. Miranda, L. Russo and O. Saydam for fruitful discussions. We also would like to thank Applied Biosystems for supplying the miRNA qRT–PCR primers and S. Idema (Neuro-oncology Research Group, Cancer Center Amsterdam) for supplying some of the serum/biopsy samples. This work was supported by the Wenner-Gren Foundation (J.S.) Stiftelsen Olle Engkvist Byggmästare (J.S.), NCI CA86355 (X.O.B. and M.S.E.), NCI CA69246 (X.O.B., M.S.E. and B.S.C.), The Goldhirs Foundation (B.S.C.) the Brain Tumor Society (A.M.K.) and the American Brain Tumor Association (T.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 608 kb)
Supplementary Information
Supplementary Table 1 (XLS 1611 kb)
Supplementary Information
Supplementary Table 2 (XLS 15529 kb)
Supplementary Information
Supplementary Table 3 (XLS 138 kb)
Rights and permissions
About this article
Cite this article
Skog, J., Würdinger, T., van Rijn, S. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10, 1470–1476 (2008). https://doi.org/10.1038/ncb1800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1800
This article is cited by
-
Current status of stem cell therapy for type 1 diabetes: a critique and a prospective consideration
Stem Cell Research & Therapy (2024)
-
Protein cargo in extracellular vesicles as the key mediator in the progression of cancer
Cell Communication and Signaling (2024)
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Molecular Cancer (2024)
-
Non-bone-derived exosomes: a new perspective on regulators of bone homeostasis
Cell Communication and Signaling (2024)
-
A novel exosome based therapeutic intervention against neuroendocrine prostate cancer
Scientific Reports (2024)