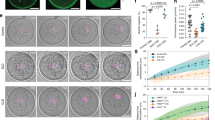
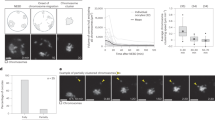
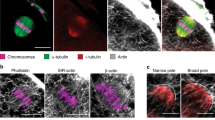
Abstract
Movement of meiosis I (MI) chromosomes from the oocyte centre to a subcortical location is the first step in the establishment of cortical polarity. This is required for two consecutive rounds of asymmetric meiotic cell divisions, which generate a mature egg and two polar bodies1. Here we use live-cell imaging and genetic and pharmacological manipulations to determine the force-generating mechanism underlying this chromosome movement. Chromosomes were observed to move toward the cortex in a pulsatile manner along a meandering path. This movement is not propelled by myosin-II-driven cortical flow but is associated with a cloud of dynamic actin filaments trailing behind the chromosomes/spindle. Formation of these filaments depends on the actin nucleation activity of Fmn2, a formin-family protein that concentrates around chromosomes through its amino-terminal region. Symmetry breaking of the actin cloud relative to chromosomes, and net chromosome translocation toward the cortex require actin turnover.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brunet, S. & Maro, B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction 130, 801–811 (2005).
Yang, H. Y., Mains, P. E. & McNally, F. J. Kinesin-1 mediates translocation of the meiotic spindle to the oocyte cortex through KCA-1, a novel cargo adapter. J. Cell Biol. 169, 447–457 (2005).
Verlhac, M. H. & Dumont, J. Interactions between chromosomes, microfilaments and microtubules revealed by the study of small GTPases in a big cell, the vertebrate oocyte. Mol. Cell Endocrinol. 282, 12–17 (2008).
Lei, Y. & Warrior, R. The Drosophila Lissencephaly1 (DLis1) gene is required for nuclear migration. Dev. Biol. 226, 57–72 (2000).
Miyazaki, A., Kato, K. H. & Nemoto, S. Role of microtubules and centrosomes in the eccentric relocation of the germinal vesicle upon meiosis reinitiation in sea-cucumber oocytes. Dev. Biol. 280, 237–247 (2005).
Longo, E., Stamato, F. M., Ferreira, R. & Tapia, O. The catalytic mechanism of serine proteases II: The effect of the protein environment in the alpha-chymotrypsin proton relay system. J. Theor. Biol. 112, 783–798 (1985).
Maro, B., Johnson, M. H., Webb, M. & Flach, G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J. Embryol. Exp. Morphol. 92, 11–32 (1986).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Rosenblatt, J., Cramer, L. P., Baum, B. & McGee, K. M. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117, 361–372 (2004).
Lenart, P. et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature 436, 812–818 (2005).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Kuo, S. C. & McGrath, J. L. Steps and fluctuations of Listeria monocytogenes during actin-based motility. Nature 407, 1026–1029 (2000).
Bernheim-Groswasser, A., Wiesner, S., Golsteyn, R. M., Carlier, M. F. & Sykes, C. The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311 (2002).
Dumont, J. et al. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev. Biol. 301, 254–265 (2007).
Simerly, C., Nowak, G., de Lanerolle, P. & Schatten, G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol. Biol. Cell 9, 2509–2525 (1998).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nature Methods 5, 605–607 (2008).
Capco, D. G., Gallicano, G. I., McGaughey, R. W., Downing, K. H. & Larabell, C. A. Cytoskeletal sheets of mammalian eggs and embryos: a lattice-like network of intermediate filaments. Cell Motil. Cytoskeleton 24, 85–99 (1993).
Leader, B. et al. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nature Cell Biol. 4, 921–928 (2002).
Pollard, T. D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36, 451–477 (2007).
Moseley, J. B. et al. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15, 896–907 (2004).
Copeland, J. W., Copeland, S. J. & Treisman, R. Homo-oligomerization is essential for F-actin assembly by the formin family FH2 domain. J. Biol. Chem. 279, 50250–50256 (2004).
Fehrenbacher, K. L., Boldogh, I. R. & Pon, L. A. Taking the A-train: actin-based force generators and organelle targeting. Trends Cell Biol. 13, 472–477 (2003).
Co, C., Wong, D. T., Gierke, S., Chang, V. & Taunton, J. Mechanism of actin network attachment to moving membranes: barbed end capture by N-WASP WH2 domains. Cell 128, 901–913 (2007).
van Oudenaarden, A. & Theriot, J. A. Cooperative symmetry-breaking by actin polymerization in a model for cell motility. Nature Cell Biol. 1, 493–499 (1999).
Kim, S. et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell 12, 571–586 (2007).
Boggio, E. M., Putignano, E., Sassoe-Pognetto, M., Pizzorusso, T. & Giustetto, M. Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE 2, e604 (2007).
Gobert, G. N. & Schatten, H. Improved ultrastructure of the desmosome-intermediate filament complex in MCF-7 breast cancer cells. J. Electron Microsc. 49, 539–544 (2000).
Egile, C. et al. Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions. PLoS Biol. 3, e383 (2005).
Verlhac, M. H., Lefebvre, C., Guillaud, P., Rassinier, P. & Maro, B. Asymmetric division in mouse oocytes: with or without Mos. Curr. Biol. 10, 1303–1306 (2000).
Acknowledgements
We thank Roland Wedlich-Soldner (Max Plank Institute, Munich) for providing F-Lifeact peptide; Philip Leder (Harvard Medical School, Boston)) for Fmn2−/− mice and full-length Fmn2 cDNA; Marie-Helene Verlhac (Universite Paris VI, Paris) for pBluescript RN3 vector; Eric Jessen (Stowers Institute, Kansa City, applying to all persons mentioned hereafter) for help in site-directed mutagenesis; Rhonda Allen for electron microscopy; Xiaoxue Fan for help with actin preparation; Praveen Suraneni and Manqi Deng for help with the mouse work; Heather Marshall and Michael Durnin for training in oocyte dissection and microinjection; and Stowers Imaging Center for assistance with live imaging; Stacie Hughes for comments on the manuscript. This work was supported by funds to R.L. from the Stowers Institute for Medical Research.
Author information
Authors and Affiliations
Contributions
H.L. and R.L. designed the experiments, analysed the data and wrote the manuscript; H.L. performed all experiments except the electron microscopy and immuno-electron microscopy experiments, which were performed by F.G.; B.R. assisted with data and statistical analyses; R.L supervised the whole project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 642 kb)
Supplementary Information
Supplementary Movie 1 (AVI 9796 kb)
Supplementary Information
Supplementary Movie 2 (AVI 6606 kb)
Supplementary Information
Supplementary Movie 3 (AVI 8677 kb)
Supplementary Information
Supplementary Movie 4 (AVI 9828 kb)
Supplementary Information
Supplementary Movie 5 (AVI 1952 kb)
Supplementary Information
Supplementary Movie 6 (AVI 3139 kb)
Rights and permissions
About this article
Cite this article
Li, H., Guo, F., Rubinstein, B. et al. Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol 10, 1301–1308 (2008). https://doi.org/10.1038/ncb1788
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1788
This article is cited by
-
Artificially decreasing cortical tension generates aneuploidy in mouse oocytes
Nature Communications (2020)
-
Dynamic organelle distribution initiates actin-based spindle migration in mouse oocytes
Nature Communications (2020)
-
Nuclear actin filaments in DNA repair dynamics
Nature Cell Biology (2019)
-
Fbxo30 regulates chromosome segregation of oocyte meiosis
Cellular and Molecular Life Sciences (2019)
-
Poly(ADP-ribose) mediates asymmetric division of mouse oocyte
Cell Research (2018)