Abstract
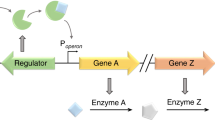
Heterologous pathways used in metabolic engineering may produce intermediates toxic to the cell. Dynamic control of pathway enzymes could prevent the accumulation of these metabolites, but such a strategy requires sensors, which are largely unknown, that can detect and respond to the metabolite. Here we applied whole-genome transcript arrays to identify promoters that respond to the accumulation of toxic intermediates, and then used these promoters to control accumulation of the intermediate and improve the final titers of a desired product. We apply this approach to regulate farnesyl pyrophosphate (FPP) production in the isoprenoid biosynthetic pathway in Escherichia coli. This strategy improved production of amorphadiene, the final product, by twofold over that from inducible or constitutive promoters, eliminated the need for expensive inducers, reduced acetate accumulation and improved growth. We extended this approach to another toxic intermediate to demonstrate the broad utility of identifying novel sensor-regulator systems for dynamic regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhang, K., Sawaya, M.R., Eisenberg, D.S. & Liao, J.C. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc. Natl. Acad. Sci. USA 105, 20653–20658 (2008).
Nakamura, C.E. & Whited, G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 14, 454–459 (2003).
Martin, V., Pitera, D., Withers, S., Newman, J. & Keasling, J. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 21, 796–802 (2003).
Basu, S., Mehreja, R., Thiberge, S., Chen, M.T. & Weiss, R. Spatiotemporal control of gene expression with pulse-generating networks. Proc. Natl. Acad. Sci. USA 101, 6355–6360 (2004).
Stricker, J. et al. A fast, robust and tunable synthetic gene oscillator. Nature 456, 516–519 (2008).
Ajikumar, P.K. et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330, 70–74 (2010).
Yuan, L.Z., Rouviere, P.E., Larossa, R.A. & Suh, W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab. Eng. 8, 79–90 (2006).
De Mey, M. et al. Promoter knock-in: a novel rational method for the fine tuning of genes. BMC Biotechnol. 10, 26 (2010).
Hammer, K., Mijakovic, I. & Jensen, P.R. Synthetic promoter libraries–tuning of gene expression. Trends Biotechnol. 24, 53–55 (2006).
Miksch, G. et al. Libraries of synthetic stationary-phase and stress promoters as a tool for fine-tuning of expression of recombinant proteins in Escherichia coli. J. Biotechnol. 120, 25–37 (2005).
Cox, R.S. III, Surette, M.G. & Elowitz, M.B. Programming gene expression with combinatorial promoters. Mol. Syst. Biol. 3, 145 (2007).
Smolke, C.D., Martin, V.J. & Keasling, J.D. Controlling the metabolic flux through the carotenoid pathway using directed mRNA processing and stabilization. Metab. Eng. 3, 313–321 (2001).
Pfleger, B.F., Pitera, D.J., Smolke, C.D. & Keasling, J.D. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat. Biotechnol. 24, 1027–1032 (2006).
Salis, H., Mirsky, E. & Voigt, C. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Holtz, W.J. & Keasling, J.D. Engineering static and dynamic control of synthetic pathways. Cell 140, 19–23 (2010).
Dunlop, M.J., Keasling, J.D. & Mukhopadhyay, A. A model for improving microbial biofuel production using a synthetic feedback loop. Syst. Synth. Biol. 4, 95–104 (2010).
Shamah, S.M., Healy, J.M. & Cload, S.T. Complex target SELEX. Acc. Chem. Res. 41, 130–138 (2008).
Fazelinia, H., Cirino, P.C. & Maranas, C.D. Extending Iterative Protein Redesign and Optimization (IPRO) in protein library design for ligand specificity. Biophys. J. 92, 2120–2130 (2007).
Winkler, W.C., Nahvi, A., Roth, A., Collins, J.A. & Breaker, R.R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428, 281–286 (2004).
Dietrich, J.A., Shis, D.L., Alikhani, A. & Keasling, J.D. Transcription factor-based screens and synthetic selections for microbial small-molecule biosynthesis. ACS Synth. Biol. 2, 47–58 (2013).
Zhang, F., Carothers, J.M. & Keasling, J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 30, 354–359 (2012).
Reiling, K.K. et al. Mono and diterpene production in Escherichia coli. Biotechnol. Bioeng. 87, 200–212 (2004).
Ro, D.K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006).
Withers, S.T., Gottlieb, S.S., Lieu, B., Newman, J.D. & Keasling, J.D. Identification of isopentenol biosynthetic genes from Bacillus subtilis by a screening method based on isoprenoid precursor toxicity. Appl. Environ. Microbiol. 73, 6277–6283 (2007).
Kizer, L., Pitera, D.J., Pfleger, B.F. & Keasling, J.D. Application of functional genomics to pathway optimization for increased isoprenoid production. Appl. Environ. Microbiol. 74, 3229–3241 (2008).
Dueber, J.E. et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 27, 753–759 (2009).
Pitera, D.J., Paddon, C.J., Newman, J.D. & Keasling, J.D. Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab. Eng. 9, 193–207 (2007).
Meshnick, S.R. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 32, 1655–1660 (2002).
Rowinsky, E.K. & Donehower, R.C. Paclitaxel (taxol). N. Engl. J. Med. 332, 1004–1014 (1995).
Krings, U. & Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 49, 1–8 (1998).
Peralta-Yahya, P.P. et al. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2, 483 (2011).
Martin, V.J., Yoshikuni, Y. & Keasling, J.D. The in vivo synthesis of plant sesquiterpenes by Escherichia coli. Biotechnol. Bioeng. 75, 497–503 (2001).
Dehal, P.S. et al. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res. 38, D396–D400 (2010).
Groisman, E.A. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183, 1835–1842 (2001).
Hinson, D.D., Chambliss, K.L., Toth, M.J., Tanaka, R.D. & Gibson, K.M. Post-translational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. J. Lipid Res. 38, 2216–2223 (1997).
Ferrandez, A., Garcia, J.L. & Diaz, E. Transcriptional regulation of the divergent paa catabolic operons for phenylacetic acid degradation in Escherichia coli. J. Biol. Chem. 275, 12214–12222 (2000).
Chin, C.S., Chubukov, V., Jolly, E.R., DeRisi, J. & Li, H. Dynamics and design principles of a basic regulatory architecture controlling metabolic pathways. PLoS Biol. 6, e146 (2008).
Wolfe, A.J. The acetate switch. Microbiol. Mol. Biol. Rev. 69, 12–50 (2005).
Redding-Johanson, A.M. et al. Targeted proteomics for metabolic pathway optimization: application to terpene production. Metab. Eng. 13, 194–203 (2011).
Hommais, F. et al. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150, 61–72 (2004).
Sayed, A.K. & Foster, J.W. A 750 bp sensory integration region directs global control of the Escherichia coli GadE acid resistance regulator. Mol. Microbiol. 71, 1435–1450 (2009).
Weber, H., Polen, T., Heuveling, J., Wendisch, V.F. & Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: sigmaS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187, 1591–1603 (2005).
Kang, Z., Wang, Q., Zhang, H. & Qi, Q. Construction of a stress-induced system in Escherichia coli for efficient polyhydroxyalkanoates production. Appl. Microbiol. Biotechnol. 79, 203–208 (2008).
Zaslaver, A. et al. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 3, 623–628 (2006).
Salis, H.M., Mirsky, E.A. & Voigt, C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Karig, D. & Weiss, R. Signal-amplifying genetic circuit enables in vivo observation of weak promoter activation in the Rhl quorum sensing system. Biotechnol. Bioeng. 89, 709–718 (2005).
Voigt, C.A. Genetic parts to program bacteria. Curr. Opin. Biotechnol. 17, 548–557 (2006).
Mahmoud, S.S. & Croteau, R.B. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc. Natl. Acad. Sci. USA 98, 8915–8920 (2001).
Shewmaker, C.K., Sheehy, J.A., Daley, M., Colburn, S. & Ke, D.Y. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J. 20, 401–412 (1999).
Grotewold, E. et al. Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10, 721–740 (1998).
Chen, C. et al. Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar. Plant Physiol. 123, 853–868 (2000).
Chen, A., Kroon, P.A. & Poulter, C.D. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 3, 600–607 (1994).
Lee, T.S. et al. BglBrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng. 5, 12 (2011).
Colantuoni, C., Henry, G., Zeger, S. & Pevsner, J. SNOMAD (Standardization and Normalization of MicroArray Data): web-accessible gene expression data analysis. Bioinformatics 18, 1540–1541 (2002).
Sturn, A., Quackenbush, J. & Trajanoski, Z. Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208 (2002).
Acknowledgements
The authors thank D. Pitera for the pADSmut plasmid and W. Holtz for the BglBrick plasmids with the lacUV5 promoter and LacIQ removed. J.A.-G. thanks Fundacion Ramon Areces for his PostDoc fellowship. This work was part of the Department of Energy Joint BioEnergy Institute (http://www.jbei.org/) supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy.
Author information
Authors and Affiliations
Contributions
R.H.D. and J.D.K. conceived the project. R.H.D. performed the growth experiments, microarrays and the construction of the responsive promoters. F.Z. constructed and analyzed the HMG-CoA biosensor. J.A.-G. analyzed the FPP sensor. E.B. performed metabolite analysis. T.S.B., A.M.R.-J. and C.J.P. performed the proteomic analysis. T.S.L., A.M. and P.D.A. directed aspects of the project. R.H.D., F.Z., J.A.-G. and J.D.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.D.K. has a financial interest in Amyris, Lygos and LS9.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–4 (PDF 1789 kb)
Rights and permissions
About this article
Cite this article
Dahl, R., Zhang, F., Alonso-Gutierrez, J. et al. Engineering dynamic pathway regulation using stress-response promoters. Nat Biotechnol 31, 1039–1046 (2013). https://doi.org/10.1038/nbt.2689
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2689
This article is cited by
-
Heterologous overproduction of oviedomycin by refactoring biosynthetic gene cluster and metabolic engineering of host strain Streptomyces coelicolor
Microbial Cell Factories (2023)
-
Biosensor-based high-throughput screening enabled efficient adipic acid production
Applied Microbiology and Biotechnology (2023)
-
Overexpression of genes by stress-responsive promoters increases protein secretion in Saccharomyces cerevisiae
World Journal of Microbiology and Biotechnology (2023)
-
Application of quorum sensing system in microbial synthesis of valuable chemicals: a mini-review
World Journal of Microbiology and Biotechnology (2022)
-
CRISPR/Cas9 mediated T7 RNA polymerase gene knock-in in E. coli BW25113 makes T7 expression system work efficiently
Journal of Biological Engineering (2021)