Abstract
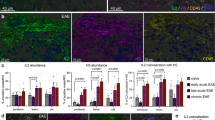
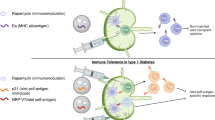
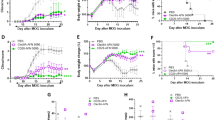
Aberrant T-cell activation underlies many autoimmune disorders, yet most attempts to induce T-cell tolerance have failed. Building on previous strategies for tolerance induction that exploited natural mechanisms for clearing apoptotic debris, we show that antigen-decorated microparticles (500-nm diameter) induce long-term T-cell tolerance in mice with relapsing experimental autoimmune encephalomyelitis. Specifically, intravenous infusion of either polystyrene or biodegradable poly(lactide-co-glycolide) microparticles bearing encephalitogenic peptides prevents the onset and modifies the course of the disease. These beneficial effects require microparticle uptake by marginal zone macrophages expressing the scavenger receptor MARCO and are mediated in part by the activity of regulatory T cells, abortive T-cell activation and T-cell anergy. Together these data highlight the potential for using microparticles to target natural apoptotic clearance pathways to inactivate pathogenic T cells and halt the disease process in autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 June 2013
In the version of this article initially published, the description of mice in the first paragraph of Online Methods was incomplete. Female SJL/J mice should have been described as SJL/JCrHsD; female BALB/c mice, as BALB/cJ mice; TCR transgenic mice expressing a TCR on the SJL/J background, expressed it on the SJL/JCrHsD background. Marco−/− mice, described as “on the BALB/c background,” should have been described as backcrossed to the BALB/cAncr1 (Charles River) background. The errors have been corrected in the HTML and PDF versions of the article.
References
Christen, U. & von Herrath, M.G. Initiation of autoimmunity. Curr. Opin. Immunol. 16, 759–767 (2004).
Chatenoud, L. & Bluestone, J.A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 7, 622–632 (2007).
Kohm, A.P., Turley, D.M. & Miller, S.D. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int. Rev. Immunol. 24, 361–392 (2005).
Miller, S.D., Turley, D.M. & Podojil, J.R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 7, 665–677 (2007).
Herold, K.C. et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin. Immunol. 132, 166–173 (2009).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Papadopoulou, A. et al. Evolution of MS lesions to black holes under DNA vaccine treatment. J. Neurol. 259, 1375–1382 (2012).
Freedman, M.S. et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 77, 1551–1560 (2011).
Smith, C.E., Eagar, T.N., Strominger, J.L. & Miller, S.D. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 102, 9595–9600 (2005).
Vanderlugt, C.L. et al. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 164, 670–678 (2000).
Turley, D.M. & Miller, S.D. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol. 178, 2212–2220 (2007).
Getts, D.R. et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol. 187, 2405–2417 (2011).
Tyner, K. & Sadrieh, N. Considerations when submitting nanotherapeutics to FDA/CDER for regulatory review. Methods Mol. Biol. 697, 17–31 (2011).
Zolnik, B.S., Gonzalez-Fernandez, A., Sadrieh, N. & Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 151, 458–465 (2010).
Viorritto, I.C., Nikolov, N.P. & Siegel, R.M. Autoimmunity versus tolerance: can dying cells tip the balance? Clin. Immunol. 122, 125–134 (2007).
Eagar, T.N. et al. CTLA-4 regulates expansion and differentiation of Th1 cells following induction of peripheral T cell tolerance. J. Immunol. 172, 7442–7450 (2004).
Fife, B.T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10, 1185–1192 (2009).
Luo, X. et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl. Acad. Sci. USA 105, 14527–14532 (2008).
Areschoug, T. & Gordon, S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11, 1160–1169 (2009).
Dahl, M. et al. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J. Clin. Invest. 117, 757–764 (2007).
Palecanda, A. et al. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189, 1497–1506 (1999).
Kraal, G., Ter Hart, H., Meelhuizen, C., Venneker, G. & Claassen, E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur. J. Immunol. 19, 675–680 (1989).
Lyszkiewicz, M. et al. SIGN-R1+MHC II+ cells of the splenic marginal zone–a novel type of resident dendritic cells. J. Leukoc. Biol. 89, 607–615 (2011).
Kang, Y.S. et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15, 177–186 (2003).
Kohm, A.P. et al. Cutting Edge: Anti-CD25 mAb injection results in the functional inactivation, not depletion of CD4+CD25+ Treg cells. J. Immunol. 176, 3301–3305 (2006).
Beverly, B., Kang, S.M., Lenardo, M.J. & Schwartz, R.H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 4, 661–671 (1992).
Smarr, C.B., Hsu, C.L., Byrne, A.J., Miller, S.D. & Bryce, P.J. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J. Immunol. 187, 5090–5098 (2011).
Martin, A.J. et al. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer Hya transplant protection by inhibiting CD154 upregulation. J. Immunol. 185, 3326–3336 (2010).
Herold, K.C. et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
Ilan, Y. et al. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J. Clin. Immunol. 30, 167–177 (2010).
Liblau, R.S. et al. High-dose soluble antigen: peripheral T-cell proliferation or apoptosis. Immunol. Rev. 142, 193–208 (1994).
Fontoura, P. & Garren, H. Multiple sclerosis therapies: molecular mechanisms and future. Results Probl. Cell Differ. 51, 259–285 (2010).
Ludvigsson, J. et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366, 433–442 (2012).
Chen, Y., Kuchroo, V.K., Inobe, J., Hafler, D.A. & Weiner, H.L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Luo, X., Herold, K.C. & Miller, S.D. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 32, 488–499 (2010).
Sherr, J., Sosenko, J., Skyler, J.S. & Herold, K.C. Prevention of type 1 diabetes: the time has come. Nat. Clin. Pract. Endocrinol. Metab. 4, 334–343 (2008).
Garren, H. et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol. 63, 611–620 (2008).
Racke, M.K. & Lovett-Racke, A.E. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J. Immunol. 186, 1887–1890 (2011).
Lalive, P.H. et al. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 25, 401–414 (2011).
Kanno, S., Furuyama, A. & Hirano, S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol. Sci. 97, 398–406 (2007).
Chen, X.W. et al. Anti-class a scavenger receptor autoantibodies from systemic lupus erythematosus patients impair phagocytic clearance of apoptotic cells by macrophages in vitro. Arthritis Res. Ther. 13, R9 (2011).
Kranich, J. et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J. Exp. Med. 205, 1293–1302 (2008).
Thelen, T. et al. The class A scavenger receptor, macrophage receptor with collagenous structure, is the major phagocytic receptor for Clostridium sordellii expressed by human decidual macrophages. J. Immunol. 185, 4328–4335 (2010).
Thakur, S.A., Hamilton, R. Jr., Pikkarainen, T. & Holian, A. Differential binding of inorganic particles to MARCO. Toxicol. Sci. 107, 238–246 (2009).
Arredouani, M.S. et al. Scavenger Receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J. Immunol. 178, 5912–5920 (2007).
Ghosh, S., Gregory, D., Smith, A. & Kobzik, L. MARCO regulates early inflammatory responses against influenza: A useful macrophage function with adverse outcome. Am. J. Respir. Cell Mol. Biol. 45, 1036–1044 (2011).
DeSilva, D.R., Urdahl, K.B. & Jenkins, M.K. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J. Immunol. 147, 3261–3267 (1991).
Bailey, S.L., Schreiner, B., McMahon, E.J. & Miller, S.D. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007).
Schreiner, B., Bailey, S.L., Shin, T., Chen, L. & Miller, S.D. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38, 2706–2717 (2008).
Acknowledgements
This work was supported by a grant from the Myelin Repair Foundation, US National Institutes of Health grants NS026543 and EB013198, Juvenile Diabetes Research Foundation grant 17-2011-343 and the Australian National Health and Medical Research Council grants 512413 and 1030897. We also thank V. Kuchroo and L. Kobchik of Harvard University for providing transgenic mice.
Author information
Authors and Affiliations
Contributions
D.R.G. and A.J.M. designed and performed the majority of the experiments, interpreted results and assisted with manuscript preparation. D.P.M., R.L.T., Z.N.H., W.T.Y. and M.T.G. performed and/or assisted with several experiments. X.L. provided reagents and advice. S.D.M. provided intellectual input, secured the funding and guided experimental design and the preparation of the manuscript with major input from M.P., N.J.C.K. and L.D.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 364 kb)
Rights and permissions
About this article
Cite this article
Getts, D., Martin, A., McCarthy, D. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol 30, 1217–1224 (2012). https://doi.org/10.1038/nbt.2434
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2434
This article is cited by
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Harnessing the liver to induce antigen-specific immune tolerance
Seminars in Immunopathology (2022)
-
Obstacles and opportunities in a forward vision for cancer nanomedicine
Nature Materials (2021)
-
Inducing immune tolerance with dendritic cell-targeting nanomedicines
Nature Nanotechnology (2021)
-
Experimental autoimmune prostatitis: different antigens induction and antigen-specific therapy
International Urology and Nephrology (2021)