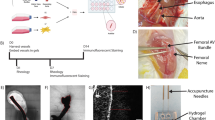
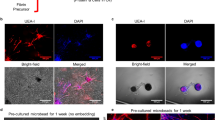
Abstract
The therapeutic potential of angiogenic growth factors has not been realized. This may be because formation of endothelial sprouts is not followed by their muscularization into vasoreactive arteries. Using microarray expression analysis, we discovered that fibroblast growth factor 9 (FGF9) was highly upregulated as human vascular smooth muscle cells (SMCs) assemble into layered cords. FGF9 was not angiogenic when mixed with tissue implants or delivered to the ischemic mouse hind limb, but instead orchestrated wrapping of SMCs around neovessels. SMC wrapping in implants was driven by sonic hedgehog–mediated upregulation of PDGFRβ. Computed tomography microangiography and intravital microscopy revealed that microvessels formed in the presence of FGF9 had enhanced capacity to receive flow and were vasoreactive. Moreover, the vessels persisted beyond 1 year, remodeling into multilayered arteries paired with peripheral nerves. This mature physiological competency was attained by targeting mesenchymal cells rather than endothelial cells, a finding that could inform strategies for therapeutic angiogenesis and tissue engineering.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Takeshita, S. et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 93, 662–670 (1994).
Gupta, R., Tongers, J. & Losordo, D.W. Human studies of angiogenic gene therapy. Circ. Res. 105, 724–736 (2009).
Losordo, D.W. & Dimmeler, S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 109, 2487–2491 (2004).
Lekas, M., Lekas, P., Latter, D.A., Kutryk, M.B. & Stewart, D.J. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: time for a re-evaluation? Curr. Opin. Cardiol. 21, 376–384 (2006).
Stewart, D.J. et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 17, 1109–1115 (2009).
Simons, M. Angiogenesis: where do we stand now? Circulation 111, 1556–1566 (2005).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Murakami, M. & Simons, M. Regulation of vascular integrity. J. Mol. Med. 87, 571–582 (2009).
Liu, H., Kennard, S. & Lilly, B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ. Res. 104, 466–475 (2009).
Tsigkou, O. et al. Engineered vascularized bone grafts. Proc. Natl. Acad. Sci. USA 107, 3311–3316 (2010).
Li, S. et al. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ. Res. 89, 517–525 (2001).
Frontini, M.J. et al. Lipid incorporation inhibits Src-dependent assembly of fibronectin and type I collagen by vascular smooth muscle cells. Circ. Res. 104, 832–841 (2009).
Murakami, M. & Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 15, 215–220 (2008).
Zhang, X. et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694–15700 (2006).
Kuzuya, M. et al. Induction of apoptotic cell death in vascular endothelial cells cultured in three-dimensional collagen lattice. Exp. Cell Res. 248, 498–508 (1999).
Koyama, N., Hart, C.E. & Clowes, A.W. Different functions of the platelet-derived growth factor-alpha and -beta receptors for the migration and proliferation of cultured baboon smooth muscle cells. Circ. Res. 75, 682–691 (1994).
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Chen, J.K., Taipale, J., Cooper, M.K. & Beachy, P.A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002).
Schechner, J.S. et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA 97, 9191–9196 (2000).
Mukouyama, Y.S., Shin, D., Britsch, S., Taniguchi, M. & Anderson, D.J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 (2002).
Lutolf, M.P. & Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55 (2005).
Benjamin, L.E., Hemo, I. & Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125, 1591–1598 (1998).
Cao, R. et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9, 604–613 (2003).
Naruo, K. et al. Novel secretory heparin-binding factors from human glioma cells (glia-activating factors) involved in glial cell growth. Purification and biological properties. J. Biol. Chem. 268, 2857–2864 (1993).
Colvin, J.S., White, A.C., Pratt, S.J. & Ornitz, D.M. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 128, 2095–2106 (2001).
Geske, M.J., Zhang, X., Patel, K.K., Ornitz, D.M. & Stappenbeck, T.S. Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development 135, 2959–2968 (2008).
Lavine, K. et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 20, 1651–1666 (2006).
White, A.C., Lavine, K. & Ornitz, D. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development 134, 3743–3752 (2007).
Hung, I.H., Yu, K., Lavine, K. & Ornitz, D. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev. Biol. 307, 300–313 (2007).
Korf-Klingebiel, M. et al. Conditional transgenic expression of fibroblast growth factor 9 in the adult mouse heart reduces heart failure mortality after myocardial infarction. Circulation 123, 504–514 (2011).
Yi, L., Domyan, E.T., Lewandoski, M. & Sun, X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev. Dyn. 238, 123–137 (2009).
Miller, D.L., Ortega, S., Bashayan, O., Basch, R. & Basilico, C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol. Cell. Biol. 20, 2260–2268 (2000).
Zhou, M. et al. Fibroblast growth factor 2 control of vascular tone. Nat. Med. 4, 201–207 (1998).
Nissen, L.J. et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J. Clin. Invest. 117, 2766–2777 (2007).
Yang, J. et al. Telomerized human microvasculature is functional in vivo. Nat. Biotechnol. 19, 219–224 (2001).
McKee, J.A. et al. Human arteries engineered in vitro. EMBO Rep. 4, 633–638 (2003).
Koike, N. et al. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138–139 (2004).
Li, S., Sims, S., Jiao, Y., Chow, L.H. & Pickering, J.G. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ. Res. 85, 338–348 (1999).
Al-Shahrour, F., Diaz-Uriarte, R. & Dopazo, J. FatiGO: a web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics 20, 578–580 (2004).
Dennis, G. Jr. et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4, 3 (2003).
Small, T.W. et al. Wilms' tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells. Circ. Res. 99, 1338–1346 (2006).
van der Veer, E. et al. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ. Res. 97, 25–34 (2005).
Pickering, J.G. et al. Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of alpha2beta1 integrin and disassembly of actin filaments. Circ. Res. 80, 627–637 (1997).
Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 (2002).
Limbourg, A. et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 4, 1737–1748 (2009).
Li, X. et al. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J. Clin. Invest. 115, 118–127 (2005).
Masocha, W. & Parvathy, S.S. Assessment of weight bearing changes and pharmacological antinociception in mice with LPS-induced monoarthritis using the Catwalk gait analysis system. Life Sci. 85, 462–469 (2009).
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (FRN-11715), Heart and Stroke Foundation of Ontario (T7081) and Lawson Health Research Institute. J.G.P. holds the Heart and Stroke Foundation of Ontario/Barnett-Ivey Chair, M.D. holds a Career Investigator Award from the Heart and Stroke Foundation of Ontario and R.G. is supported by a New Investigator Award from the Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Contributions
M.J.F. undertook experimentation and contributed to the manuscript preparation. Z.N. performed animal surgeries and tissue immunohistochemistry. R.G. contributed to the intravital microscopy experiments and laser Doppler perfusion studies and undertook gait analyses. M.D. undertook micro-CT angiography and its analysis. C.O. and M.N.R. performed the microarray experiments and cell proliferation and migration studies and C.O. also contributed to the laser Doppler flow analyses. O.A. contributed to the telomere analyses. H.Y. undertook the FGFR activation studies and contributed to manuscript preparation. C.G.E. provided technical support and conceptual advice for intravital experiments. J.G.P. conceived and designed the study and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.G.P., M.J.F. and Z.N. hold a patent relating to the use of FGF9 in the context of the findings in this report.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 4216 kb)
Supplementary Video 1
Vasomotion of control neovessel (MOV 3379 kb)
Supplementary Video 2
Vasomotion of FGF9-modified neovessel (MOV 3225 kb)
Rights and permissions
About this article
Cite this article
Frontini, M., Nong, Z., Gros, R. et al. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol 29, 421–427 (2011). https://doi.org/10.1038/nbt.1845
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1845
This article is cited by
-
Circulating inflammatory biomarkers and risk of intracranial aneurysm: a Mendelian randomization study
European Journal of Medical Research (2024)
-
Embryo-endometrial interaction associated with the location of the embryo during the mobility phase in mares
Scientific Reports (2024)
-
Umbilical cord blood exosomes from very preterm infants with bronchopulmonary dysplasia aggravate lung injury in mice
Scientific Reports (2023)
-
Human endometrium-derived stem cell improves cardiac function after myocardial ischemic injury by enhancing angiogenesis and myocardial metabolism
Stem Cell Research & Therapy (2021)
-
Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells
Scientific Reports (2017)