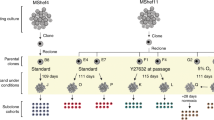
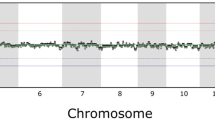
Abstract
The International Stem Cell Initiative analyzed 125 human embryonic stem (ES) cell lines and 11 induced pluripotent stem (iPS) cell lines, from 38 laboratories worldwide, for genetic changes occurring during culture. Most lines were analyzed at an early and late passage. Single-nucleotide polymorphism (SNP) analysis revealed that they included representatives of most major ethnic groups. Most lines remained karyotypically normal, but there was a progressive tendency to acquire changes on prolonged culture, commonly affecting chromosomes 1, 12, 17 and 20. DNA methylation patterns changed haphazardly with no link to time in culture. Structural variants, determined from the SNP arrays, also appeared sporadically. No common variants related to culture were observed on chromosomes 1, 12 and 17, but a minimal amplicon in chromosome 20q11.21, including three genes expressed in human ES cells, ID1, BCL2L1 and HM13, occurred in >20% of the lines. Of these genes, BCL2L1 is a strong candidate for driving culture adaptation of ES cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Baker, D.E. et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 25, 207–215 (2007).
Draper, J.S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat. Biotechnol. 22, 53–54 (2004).
Mitalipova, M.M. et al. Preserving the genetic integrity of human embryonic stem cells. Nat. Biotechnol. 23, 19–20 (2005).
Hoffman, L.M. & Carpenter, M.K. Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 23, 699–708 (2005).
Maitra, A. et al. Genomic alterations in cultured human embryonic stem cells. Nat. Genet. 37, 1099–1103 (2005).
Buzzard, J.J., Gough, N.M., Crook, J.M. & Colman, A. Karyotype of human ES cells during extended culture. Nat. Biotechnol. 22, 381–382, author reply 382 (2004).
Caisander, G. et al. Chromosomal integrity maintained in five human embryonic stem cell lines after prolonged in vitro culture. Chromosome Res. 14, 131–137 (2006).
Inzunza, J. et al. Comparative genomic hybridization and karyotyping of human embryonic stem cells reveals the occurrence of an isodicentric X chromosome after long-term cultivation. Mol. Hum. Reprod. 10, 461–466 (2004).
Rosler, E.S. et al. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev. Dyn. 229, 259–274 (2004).
Lefort, N. et al. Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat. Biotechnol. 26, 1364–1366 (2008).
Spits, C. et al. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat. Biotechnol. 26, 1361–1363 (2008).
Mayshar, Y. et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 7, 521–531 (2010).
Wang, N., Trend, B., Bronson, D.L. & Fraley, E.E. Nonrandom abnormalities in chromosome 1 in human testicular cancers. Cancer Res. 40, 796–802 (1980).
Atkin, N.B. & Baker, M.C. Specific chromosome change, i(12p), in testicular tumours? Lancet 320, 1349 (1982).
Rodriguez, E. et al. Molecular cytogenetic analysis of i(12p)-negative human male germ cell tumors. Genes Chromosom. Cancer 8, 230–236 (1993).
Skotheim, R.I. et al. New insights into testicular germ cell tumorigenesis from gene expression profiling. Cancer Res. 62, 2359–2364 (2002).
Mostert, M. et al. Comparative genomic and in situ hybridization of germ cell tumors of the infantile testis. Lab. Invest. 80, 1055–1064 (2000).
Schneider, D.T. et al. Genetic analysis of childhood germ cell tumors with comparative genomic hybridization. Klin. Padiatr. 213, 204–211 (2001).
Looijenga, L.H. et al. Comparative genomic hybridization of microdissected samples from different stages in the development of a seminoma and a non-seminoma. J. Pathol. 191, 187–192 (2000).
Longo, L., Bygrave, A., Grosveld, F.G. & Pandolfi, P.P. The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimaerism. Transgenic Res. 6, 321–328 (1997).
Liu, X. et al. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev. Dyn. 209, 85–91 (1997).
Zody, M.C. et al. DNA sequence of human chromosome 17 and analysis of rearrangement in the human lineage. Nature 440, 1045–1049 (2006).
Werbowetski-Ogilvie, T.E. et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat. Biotechnol. 27, 91–97 (2009).
Narva, E. et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat. Biotechnol. 28, 371–377 (2010).
Allegrucci, C. et al. Restriction landmark genome scanning identifies culture-induced DNA methylation instability in the human embryonic stem cell epigenome. Hum. Mol. Genet. 16, 1253–1268 (2007).
Calvanese, V. et al. Cancer genes hypermethylated in human embryonic stem cells. PLoS ONE 3, e3294 (2008).
Enver, T. et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum. Mol. Genet. 14, 3129–3140 (2005).
Rugg-Gunn, P.J., Ferguson-Smith, A.C. & Pedersen, R.A. Epigenetic status of human embryonic stem cells. Nat. Genet. 37, 585–587 (2005).
Adewumi, O. et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 25, 803–816 (2007).
Rugg-Gunn, P.J., Ferguson-Smith, A.C. & Pedersen, R.A. Status of genomic imprinting in human embryonic stem cells as revealed by a large cohort of independently derived and maintained lines. Hum. Mol. Genet. 16 Spec No. 2, R243–R251 (2007).
Kim, K.P. et al. Gene-specific vulnerability to imprinting variability in human embryonic stem cell lines. Genome Res. 17, 1731–1742 (2007).
Andrews, P.W. et al. The International Stem Cell Initiative: toward benchmarks for human embryonic stem cell research. Nat. Biotechnol. 23, 795–797 (2005).
Olariu, V. et al. Modeling the evolution of culture-adapted human embryonic stem cells. Stem Cell Res. 4, 50–56 (2010).
Martin, G.R. & Evans, M.J. The morphology and growth of a pluripotent teratocarcinoma cell line and its derivatives in tissue culture. Cell 2, 163–172 (1974).
Andrews, P.W., Bronson, D.L., Benham, F., Strickland, S. & Knowles, B.B. A comparative study of eight cell lines derived from human testicular teratocarcinoma. Int. J. Cancer 26, 269–280 (1980).
Chambers, I. et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 (2003).
Mitsui, K. et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 (2003).
Darr, H., Mayshar, Y. & Benvenisty, N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development 133, 1193–1201 (2006).
Korkola, J.E. et al. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 66, 820–827 (2006).
Widschwendter, M. et al. Epigenetic stem cell signature in cancer. Nat. Genet. 39, 157–158 (2007).
Frazer, K.A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Li, J.Z. et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science 319, 1100–1104 (2008).
Abdulla, M.A. et al. Mapping human genetic diversity in Asia. Science 326, 1541–1545 (2009).
Pritchard, J.K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Novembre, J. et al. Genes mirror geography within Europe. Nature 456, 98–101 (2008).
Laurent, L.C. et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8, 106–118 (2011).
Wang, K. et al. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 17, 1665–1674 (2007).
Assou, S. et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 25, 961–973 (2007).
Peng, J.C. et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139, 1290–1302 (2009).
Nottke, A., Colaiacovo, M.P. & Shi, Y. Developmental roles of the histone lysine demethylases. Development 136, 879–889 (2009).
Morin, R.D. et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 18, 610–621 (2008).
Bai, H. et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. (Amst.) (in the press).
Lee, T.I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Hussein, S.M. et al. Copy number variation and selection during reprogramming to pluripotency. Nature 471, 58–62 (2011).
Mosher, J.T. et al. Lack of population diversity in commonly used human embryonic stem-cell lines. N. Engl. J. Med. 362, 183–185 (2010).
Laurent, L.C. et al. Restricted ethnic diversity in human embryonic stem cell lines. Nat. Methods 7, 6–7 (2010).
Wu, H. et al. Copy number variant analysis of human embryonic stem cells. Stem Cells 26, 1484–1489 (2008).
Chia, N.Y. et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468, 316–320 (2010).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Ardehali, R. et al. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc. Natl. Acad. Sci. USA 108, 3282–3287 (2011).
Martins-Taylor, K. et al. Recurrent copy number variations in human induced pluripotent stem cells. Nat. Biotechnol. 29, 488–491 (2011).
Deloukas, P. et al. The DNA sequence and comparative analysis of human chromosome 20. Nature 414, 865–871 (2001).
Shaw, C.J. & Lupski, J.R. Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum. Mol. Genet. 13 Spec No 1, R57–R64 (2004).
Misceo, D. et al. Evolutionary history of chromosome 20. Mol. Biol. Evol. 22, 360–366 (2005).
The International Stem Cell Banking Initiative. Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev. 5, 301–314 (2009).
Hook, E.B. Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am. J. Hum. Genet. 29, 94–97 (1977).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Bibikova, M. et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res. 16, 383–393 (2006).
Acknowledgements
The International Stem Cell Initiative is funded by The International Stem Cell Forum. The authors would like to acknowledge the following: Medical Research Council, UK (P.W.A., H.M.); Mohammad Pakzad & Adeleh Taei, Royan Institute (H.B., G.H.S.); California Institute for Regenerative Medicine (CIRM) (E.C., P.W.L.); Institute of Medical Biology, A*STAR, Singapore (J.M.C.); Ministry of Education, Youth and Sports of the Czech Republic (P.D., A.H.); Stem Cell Research Center of the 21st Century Frontier Research Program, Ministry of Education, Science & Technology, Republic of Korea (SC-1140) (D.R.L., S.K.O.); Ministry of Science and Technology of China (863 program 2006AA02A102) (L.G.); Swedish Research Council, Cellartis (O.H.); Department of Biotechnology, Government of India, UK-India Education and Research Initiative and the Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India (M.I.); Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, Leading Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) of the Japan Society for the Promotion of Science (JSPS), Grants in-Aid for Scientific Research of JSPS and MEXT (T.I., S.Y., K.T.); Swiss National Science Foundation (grant no. 4046-114410) (M.J.); Shanghai Science and Technology Developmental Foundation (06DJ14001), Chinese Ministry of Science and Technology (2007CB948004) (Y.J.); funding from the North West Science Fund, UK (S.K.); One North East Regional Developmental Agency, Medical Research Council, UK, Newcastle University (M.L.); research funding from the Australian Stem Cell Centre (A.L.L.); The Netherlands Proteomics Consortium grant T4-3 (C.M.); Stem Cell Network, Canada (A.N.); National BioResource Project, MEXT, Japan (N.N.); Singapore Stem Cell Consortium (SSCC) & the Agency for Science Technology and Research (A*STAR) (S.K.W.O., P.R.) and the Genome Institute of Singapore Core Genotyping Lab (P.R.); Academy of Finland, Sigrid Juselius Foundation (T.O.); Conselho Nacional de Desenvolvimento Científico e Tecnológico/Departamento de Ciência e Tecnologia do Ministério da Saúde (CNPq/MS/DECIT), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (L.V.P.); supported by the kind donation of Judy and Sidney Swartz (B.R.); financial support from the Faculty of Medicine, University of New South Wales (UNSW) and the National Health and Medical Research Council (NHMRC) Program Grant no. 568969 (Perminder Sachdev), South Eastern Sydney and Illawarra Area Health Service (SEIAHS) for making hES cell line Endeavour-2 available for this study, and H. Chung and J. Kim for their help in preparing the samples (K.S.); Academy of Finland (grant 218050), the Competitive Research Funding of the Tampere University Hospital (grant 9F217) (H. Skottman).
Author information
Authors and Affiliations
Consortia
Contributions
Project coordination: P.W.A. Cytogenetic analyses: D.B., A.D., E.M., K.D.M. and T.G.-L. Molecular karyotyping by SNP BeadChip: P.R. DNA methylation arrays: R.M.B. and P.W.L. Administration and data curation: A. Ford and P.J.G. Data analysis and manuscript drafting: P.W.A., S.A., D.B., N.B., R.M.B., P.J.G., K.H., L.H., B.B.K., Y. Mayshar, S.K.W.O., M.F.P. and P.R. The scientific management of the ISCI project was provided by a steering committee comprising: P.W.A., N.B., B.B.K., S.K.W.O., M.F.P., J.R. and G.N.S. Sample contribution: A. Colman, A. Robins, A. Hampl, A. Bosman, A.M. Fraga, A. Nagy, A.B.H. Choo, A.L. Laslett, A. Feki, A. Kuliev, A. Kresentia Irwanto, B. Reubinoff, B. Sun, C. Denning, C. Mummery, C. Li, C. Olson, C. Spits, D. Ben-Yosef, D. Collins, D.J. Weisenberger, D. Ryul Lee, D. Ward-van Oostwaard, E. Chiao, E. Sherrer, Fei Pan, F. Holm, G. Anyfantis, G.Q. Daley, G.H. Salekdeh, G. Selva Raj, G. Caisander, H. Gourabi, H. Moore, H. Skottman, H. Suemori, H. Baharvand, H. Shen, I. Mateizel, In-Hyun Park, J. Sheik Mohamed, J. Downie, J. Eun Lee, J.M. Crook, J. Chen, J. Hyllner, J.-C. Biancotti, J. Baker, K. Sermon, K. Amps, K. Narwani, K. Takahashi, K. Sidhu, L. Ge, L.S. Lim, L. Young, Q. Zhou, L. Guangxiu, L.V. Pereira, L. Armstrong, M. Lako, M.S. Inamdar, M.A. Lagarkova, M.B. Munoz, M. Mileikovsky, M.V. Camarasa, M. Jaconi, M. Gropp, N. Lavon, N. Strelchenko, N. Nakatsuji, O. Kopper, O. Hovatta, O. Qi, P. Venu, P.A. De Sousa, P. Dvorak, R. Strehl, R. Suuronen, S. Kiselev, S. Yong Moon, S. Yamanaka, S. Sivarajah, S. Beil, S.L. Minger, S.K.W. Oh, S. Pells, S. Kyung Oh, S. Kimber, T. Miyazaki, T.E. Ludwig, T. Ishii, T.C. Schulz, T. Otonkoski, T. Tuuri, T. Frumkin, V. Kukharenko, V. Fox, W. Herath, Y. Jin, Y. Min Choi, Y. Ma, Y. Wu and Y. Verlinsky.
Ethics declarations
Competing interests
G. Caisander, J. Hyllner and R. Strehl are employees of Cellartis AB. E. Chiao is an employee of Hoffmann-LaRoche. D. Collins and J. Downie are employees of Roslin Cells Ltd. P.A. De Sousa is CSO of Roslin Cells Ltd. S.L. Minger is an employee of GE Healthcare. B. Reubinoff holds shares and is the CSO of CellCure Neurosciences Ltd. A. Robins, T.C. Schulz and E. Sherrer are employees of Viacyte. S. Yamanaka is a member of the scientific advisory board of iPierian Inc and iPS Academia Japan, Inc. without salary
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Notes 1 and 2. (PDF 649 kb)
Supplementary Table 1
Full details of cell line samples provided. (XLS 265 kb)
Supplementary Table 2
ES-associated structural variants. (XLSX 224 kb)
Supplementary Table 3
The extent of DNA methylation changes in ES cell lines in relation to the difference between early and late passage levels. (PDF 40 kb)
Supplementary Table 4
Polycomb array manifest. (XLS 431 kb)
Supplementary Table 5
Cell line availability. (XLSX 24 kb)
Supplementary Data Set 1
.bed file of all LOH calls for all samples in the ISCI-2 sample set (XLS 7081 kb)
Supplementary Data Set 2
LOH calls for all samples in the ISCI-2 sample set (ZIP 3089 kb)
Supplementary Data Set 3
.bed file of CNV calls from karyotypically normal samples in the ISCI-2 sample set. (TXT 6779 kb)
Supplementary Data Set 4
Complete β-scores (methylation level) of all ISCI-2 samples (ZIP 1435 kb)
Rights and permissions
About this article
Cite this article
The International Stem Cell Initiative. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol 29, 1132–1144 (2011). https://doi.org/10.1038/nbt.2051
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2051
This article is cited by
-
Epigenetic repression of CHCHD2 enhances survival from single cell dissociation through attenuated Rho A kinase activity
Cellular and Molecular Life Sciences (2024)
-
Trisomy 12 compromises the mesendodermal differentiation propensity of human pluripotent stem cells
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Liver organoid culture methods
Cell & Bioscience (2023)
-
Suspension culture improves iPSC expansion and pluripotency phenotype
Stem Cell Research & Therapy (2023)
-
Challenges in the clinical advancement of cell therapies for Parkinson’s disease
Nature Biomedical Engineering (2023)