Abstract
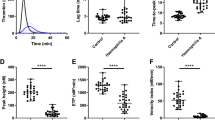
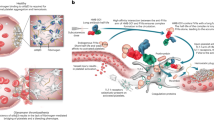
Effective therapies are needed to control excessive bleeding in a range of clinical conditions. We improve hemostasis in vivo using a conformationally pliant variant of coagulation factor Xa (FXaI16L) rendered partially inactive by a defect in the transition from zymogen to active protease1,2. Using mouse models of hemophilia, we show that FXaI16L has a longer half-life than wild-type FXa and does not cause excessive activation of coagulation. Once clotting mechanisms are activated to produce its cofactor FVa, FXaI16L is driven to the protease state and restores hemostasis in hemophilic animals upon vascular injury. Moreover, using human or murine analogs, we show that FXaI16L is more efficacious than FVIIa, which is used to treat bleeding in hemophilia inhibitor patients3. FXaI16L may provide an effective strategy to enhance blood clot formation and act as a rapid pan-hemostatic agent for the treatment of bleeding conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Toso, R., Zhu, H. & Camire, R.M. The conformational switch from the factor X zymogen to protease state mediates exosite expression and prothrombinase assembly. J. Biol. Chem. 283, 18627–18635 (2008).
Bunce, M.W., Toso, R. & Camire, R.M. Zymogen-like factor Xa variants restore thrombin generation and effectively bypass the intrinsic pathway in vitro. Blood 117, 290–298 (2011).
Hedner, U. Mechanism of action, development and clinical experience of recombinant FVIIa. J. Biotechnol. 124, 747–757 (2006).
Roberts, H.R. & Ma,, A.D. Overview of inherited hemorrhagic disorders. in Hemostasis and Thrombosis: Basic Principles and Clinical Practice. (eds. Colman, R.W., Marder, V.J., Clowes, A.W., Gerorge, J.N. & Goldhaber, S.Z.) 877–885 (Lippincott Williams & Wilkins, Philadelphia, 2006).
Mann, K.G., Nesheim, M.E., Church, W.R., Haley, P.E. & Krishnaswamy, S. Surface dependent reactions of the vitamin K-dependent enzyme complexes. Blood 76, 1–16 (1990).
Mannucci, P.M. Back to the future: a recent history of haemophilia treatment. Haemophilia 14 (suppl. 3), 10–18 (2008).
DiMichele, D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br. J. Haematol. 138, 305–315 (2007).
Darby, S.C. et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–1999. J. Thromb. Haemost. 2, 1047–1054 (2004).
Turecek, P.L., Varadi, K., Gritsch, H. & Schwarz, H.P. FEIBA: mode of action. Haemophilia 10, 3–9 (2004).
Berntorp, E. Differential response to bypassing agents complicates treatment in patients with haemophilia and inhibitors. Haemophilia 15, 3–10 (2009).
Escobar, M.A. Health economics in haemophilia: a review from the clinician's perspective. Haemophilia 16 (suppl. 3), 29–34 (2010).
Monroe, D.M., Hoffman, M., Oliver, J.A. & Roberts, H.R. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br. J. Haematol. 99, 542–547 (1997).
Butenas, S., Brummel, K.E., Bouchard, B.A. & Mann, K.G. How factor VIIa works in hemophilia. J. Thromb. Haemost. 1, 1158–1160 (2003).
Gitel, S.N., Medina, V.M. & Wessler, S. Inhibition of human activated Factor X by antithrombin III and alpha 1-proteinase inhibitor in human plasma. J. Biol. Chem. 259, 6890–6895 (1984).
Giles, A.R., Nesheim, M.E. & Mann, K.G. Studies of Factors V and VIII:C in an animal model of disseminated intravascular coagulation. J. Clin. Invest. 74, 2219–2225 (1984).
Giles, A.R., Mann, K.G. & Nesheim, M.E. A combination of factor Xa and phosphatidylcholine-phosphatidylserine vesicles bypasses factor VIII in vivo. Br. J. Haematol. 69, 491–497 (1988).
Khan, A.R. & James, M.N.G. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 7, 815–836 (1998).
Bode, W. et al. The refined 1.9 Å crystal structure of human α-thrombin: Interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Trp insertion segment. EMBO J. 8, 3467–3475 (1989).
Tchaikovski, S.N., van Vlijmen, B.J., Rosing, J. & Tans, G. Development of a calibrated automated thrombography based thrombin generation test in mouse plasma. J. Thromb. Haemost. 5, 2079–2086 (2007).
Schlachterman, A. et al. Factor V Leiden improves in vivo hemostasis in murine hemophilia models. J. Thromb. Haemost. 3, 2730–2737 (2005).
Turecek, P.L. et al. Factor VIII inhibitor-bypassing agents act by inducing thrombin generation and can be monitored by a thrombin generation assay. Pathophysiol. Haemost. Thromb. 33, 16–22 (2003).
Aljamali, M.N. et al. Long-term expression of murine activated factor VII is safe, but elevated levels cause premature mortality. J. Clin. Invest. 118, 1825–1834 (2008).
Margaritis, P. et al. Catalytic domain modification and viral gene delivery of activated factor VII confers hemostasis at reduced expression levels and vector doses in vivo. Blood 117, 3974–3982 (2011).
Weeterings, C., Lisman, T. & de Groot, P.G. Tissue factor-independent effects of recombinant factor VIIa on hemostasis. Semin. Hematol. 45, S12–S15 (2008).
Monroe, D.M., Hoffman, M. & Roberts, H.R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 22, 1381–1389 (2002).
Tranholm, M. et al. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood 102, 3615–3620 (2003).
Petersen, L.C. et al. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb. Res. 116, 75–85 (2005).
Tracy, P.B., Eide, L.L., Bowie, E.J.W. & Mann, K.G. Radioimmunoassay of factor V in human plasma and platelets. Blood 60, 59–63 (1982).
Moss, J., Scharling, B., Ezban, M. & Moller, S.T. Evaluation of the safety and pharmacokinetics of a fast-acting recombinant FVIIa analogue, NN1731, in healthy male subjects. J. Thromb. Haemost. 7, 299–305 (2009).
Weiler-Guettler, H. et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J. Clin. Invest. 101, 1983–1991 (1998).
Neyman, M., Gewirtz, J. & Poncz, M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood 112, 1101–1108 (2008).
Lin, H.F., Maeda, N., Smithies, O., Straight, D.L. & Stafford, D.W. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 90, 3962–3966 (1997).
Kung, S.H. et al. Human factor IX corrects the bleeding diathesis of mice with hemophilia B. Blood 91, 784–790 (1998).
Margaritis, P. et al. Novel therapeutic approach for hemophilia using gene delivery of an engineered secreted activated Factor VII. J. Clin. Invest. 113, 1025–1031 (2004).
Sambrano, G.R., Weiss, E.J., Zheng, Y.W., Huang, W. & Coughlin, S.R. Role of thrombin signaling in platelets in haemostasis and thrombosis. Nature 413, 74–78 (2001).
Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B.C. & Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175–1181 (2002).
Acknowledgements
This work was supported in part by US National Institutes of Health grant P01 HL-74124, Project 2; research funding from Pfizer (to R.M.C.); and the Judith Graham Pool Postdoctoral Research Fellowship, National Hemophilia Foundation (to L.I.). We would like to thank L. Albert and B. Carito (Pfizer), and A. Hannan (Children's Hospital of Philadelphia) for their technical assistance. We are also grateful to S. Krishnaswamy (Children's Hospital of Philadelphia/University of Pennsylvania) for useful suggestions and critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
L.I. designed, coordinated and performed experiments; R.T., P.M., G.P., H.K., A.S., J.-H.L. and K.M.S. performed experiments; R.R.-M. coordinated and performed experiments; V.C. designed and analyzed experiments; D.D.P. interpreted data; J.F.T., D.V.E., V.R.A. and R.M.C. designed experiments and analyzed data. L.I. and R.M.C. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.M.C. receives licensing fees and research funding from Pfizer. V.C., D.D.P., R.R.-M., K.M.S., D.V.E. and J.F.T. are employees of Pfizer, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–8 (PDF 573 kb)
Supplementary Movie 1
Representative movie depicting thrombus formation following laser injury in a wild-type mouse (Balb/c). Brightfield images of the cremaster muscle along with accumulating platelets and fibrin are shown. Platelets (red) were detected by an Alexa555-labeled rat anti- CD41 F(ab)2 and fibrin (green) with Alexa488-labeled anti-fibrin antibody; areas of overlap are depicted by yellow. A 10 μm scale bar is shown in the lower left corner and a time stamp in the upper right corner. For convenience, the speed of the movie is increased by 5-fold. Further details of the methodology can be found in Methods. (MOV 4026 kb)
Supplementary Movie 2
Representative movie depicting thrombus formation following laser injury in an HB mouse (Balb/c) treated with FXaI16L (10 μg/kg) prior to laser injury. Platelets and fibrin accumulation were detected as described in Supplemental Movie 1 and in Methods. (MOV 5221 kb)
Supplementary Movie 3
Representative movie depicting thrombus formation following laser injury in an HB mouse (Balb/c) treated with FXaI16L (30 μg/kg). In this experiment, the animal was injured and monitored for approximately 3.5 min. After this observation period, FXaI16L was infused via a jugular vein cannulus and thrombus formation was immediately monitored. The animal was not repositioned or reinjured. Platelets and fibrin accumulation were detected as described in Supplemental Movie 1 and in Methods. (MOV 15529 kb)
Rights and permissions
About this article
Cite this article
Ivanciu, L., Toso, R., Margaritis, P. et al. A zymogen-like factor Xa variant corrects the coagulation defect in hemophilia. Nat Biotechnol 29, 1028–1033 (2011). https://doi.org/10.1038/nbt.1995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1995
This article is cited by
-
Blood coagulation factor X: molecular biology, inherited disease, and engineered therapeutics
Journal of Thrombosis and Thrombolysis (2021)
-
Role of the I16-D194 ionic interaction in the trypsin fold
Scientific Reports (2019)
-
A rapid pro-hemostatic approach to overcome direct oral anticoagulants
Nature Medicine (2016)
-
New and Emerging Agents for the Treatment of Hemophilia: Focus on Extended Half-Life Recombinant Clotting Proteins
Drugs (2015)
-
An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia
Nature Medicine (2015)