Abstract
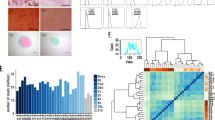
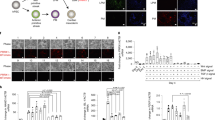
We report a chemically defined, efficient, scalable and reproducible protocol for differentiation of human embryonic stem cells (hESCs) toward chondrocytes. HESCs are directed through intermediate developmental stages using substrates of known matrix proteins and chemically defined media supplemented with exogenous growth factors. Gene expression analysis suggests that the hESCs progress through primitive streak or mesendoderm to mesoderm, before differentiating into a chondrocytic culture comprising cell aggregates. At this final stage, 74% (HUES1 cells) and up to 95–97% (HUES7 and HUES8 cells) express the chondrogenic transcription factor SOX9. The cell aggregates also express cell surface CD44 and aggrecan and deposit a sulfated glycosaminoglycan and cartilage-specific collagen II matrix, but show very low or no expression of genes and proteins associated with nontarget cell types. Our protocol should facilitate studies of chondrocyte differentiation and of cell replacement therapies for cartilage repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goldring, M.B. & Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Hardingham, T.E. Articular cartilage. in Oxford Textbook of Rheumatology (eds. Maddison, P.J., Isenberg, D.A., Woo, P. & Glass, D.N.) 325–334, (Oxford University Press, Oxford, UK, 2004).
Hardingham, T.E., Oldershaw, R.A. & Tew, S.R. Cartilage, SOX9 and Notch signals in chondrogenesis. J. Anat. 209, 469–480 (2006).
Murry, C.E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
De Sousa, P.A. et al. Clinically failed eggs as a source of normal human embryo stem cells. Stem Cell Res. 2, 188–197 (2009).
Kawaguchi, J., Mee, P.J. & Smith, A.G. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 36, 758–769 (2005).
Bigdeli, N. et al. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells 27, 1812–1821 (2009).
Boyd, N.L., Robbins, K.R., Dhara, S.K., West, F.D. & Stice, S.L. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng. Part A 15, 1897–1907 (2009).
Hoben, G.M., Willard, V.P. & Athanasiou, K.A. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 18, 283–292 (2009).
Koay, E.J., Hoben, G.M. & Athanasiou, K.A. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells 25, 2183–2190 (2007).
Kramer, J. et al. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech. Dev. 92, 193–205 (2000).
Lee, E.J. et al. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng. Part A 16, 705–715 (2009).
Sui, Y., Clarke, T. & Khillan, J.S. Limb bud progenitor cells induce differentiation of pluripotent embryonic stem cells into chondrogenic lineage. Differentiation 71, 578–585 (2003).
Vats, A. et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 12, 1687–1697 (2006).
Yang, Z., Sui, L., Toh, W.S., Lee, E.H. & Cao, T. Stage-dependent effect of TGF-β1 on chondrogenic differentiation of human embryonic stem cells. Stem Cells Dev. 18, 929–940 (2009).
zur Nieden, N.I., Kempka, G., Rancourt, D.E. & Ahr, H.J. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev. Biol. 5, 1 (2005).
Lian, Q. et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 25, 425–436 (2007).
Nakagawa, T., Lee, S.Y. & Reddi, A.H. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor β1. Arthritis Rheum. 60, 3686–3692 (2009).
D'Amour, K.A. et al. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006).
Hay, D.C. et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26, 894–902 (2008).
Laflamme, M.A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Yan, Y. et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790 (2005).
Nistor, G.I., Totoiu, M.O., Haque, N., Carpenter, M.K. & Keirstead, H.S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385–396 (2005).
Winslow, B.B., Takimoto-Kimura, R. & Burke, A.C. Global patterning of the vertebrate mesoderm. Dev. Dyn. 236, 2371–2381 (2007).
Gadue, P., Huber, T.L., Paddison, P.J. & Keller, G.M. Wnt and TGF-βbeta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 16806–16811 (2006).
Sumi, T., Tsuneyoshi, N., Nakatsuji, N. & Suemori, H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/βbeta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 (2008).
Tada, S. et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363–4374 (2005).
Izumi, N., Era, T., Akimaru, H., Yasunaga, M. & Nishikawa, S. Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells 25, 1664–1674 (2007).
Wilkinson, D.G., Bhatt, S. & Herrmann, B.G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343, 657–659 (1990).
Ema, M., Takahashi, S. & Rossant, J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 107, 111–117 (2006).
Era, T. et al. Multiple mesoderm subsets give rise to endothelial cells, whereas hematopoietic cells are differentiated only from a restricted subset in embryonic stem cell differentiation culture. Stem Cells 26, 401–411 (2008).
Faloon, P. et al. Basic fibroblast growth factor positively regulates hematopoietic development. Development 127, 1931–1941 (2000).
Zhang, P. et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111, 1933–1941 (2008).
Hall, B.K. & Miyake, T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22, 138–147 (2000).
Akiyama, H., Chaboissier, M.C., Martin, J.F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002).
Lefebvre, V., Behringer, R.R. & de Crombrugghe, B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage 9 Suppl. A, S69–S75 (2001).
Lefebvre, V., Huang, W., Harley, V.R., Goodfellow, P.N. & de Crombrugghe, B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro α1(II) collagen gene. Mol. Cell. Biol. 17, 2336–2346 (1997).
Baxter, M.A. et al. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 3, 28–38 (2009).
Eastham, A.M. et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 67, 11254–11262 (2007).
Kispert, A., Herrmann, B.G., Leptin, M. & Reuter, R. Homologs of the mouse Brachyury gene are involved in the specification of posterior terminal structures in Drosophila, Tribolium, and Locusta. Genes Dev. 8, 2137–2150 (1994).
Kubo, A. et al. Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662 (2004).
Takenaga, M., Fukumoto, M. & Hori, Y. Regulated nodal signaling promotes differentiation of the definitive endoderm and mesoderm from ES cells. J. Cell Sci. 120, 2078–2090 (2007).
Betsholtz, C., Karlsson, L. & Lindahl, P. Developmental roles of platelet-derived growth factors. Bioessays 23, 494–507 (2001).
Ducy, P. Cbfa1: a molecular switch in osteoblast biology. Dev. Dyn. 219, 461–471 (2000).
Rosen, E.D. The transcriptional basis of adipocyte development. Prostaglandins Leukot. Essent. Fatty Acids 73, 31–34 (2005).
Schweitzer, R. et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 (2001).
Pelttari, K. et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 54, 3254–3266 (2006).
Coipeau, P. et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy 11, 584–594 (2009).
Murphy, J.M. et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 46, 704–713 (2002).
Sachlos, E. & Auguste, D.T. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials 29, 4471–4480 (2008).
Izzi, L. et al. Foxh1 recruits Gsc to negatively regulate Mixl1 expression during early mouse development. EMBO J. 26, 3132–3143 (2007).
McLean, A.B. et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 25, 29–38 (2007).
Hatakeyama, Y., Tuan, R.S. & Shum, L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J. Cell. Biochem. 91, 1204–1217 (2004).
Pacifici, M., Koyama, E. & Iwamoto, M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res. C Embryo Today 75, 237–248 (2005).
Osafune, K. et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 26, 313–315 (2008).
Tew, S.R. & Hardingham, T.E. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J. Biol. Chem. 281, 39471–39479 (2006).
Grover, J. & Roughley, P.J. Expression of cell-surface proteoglycan mRNA by human articular chondrocytes. Biochem. J. 309, 963–968 (1995).
Oldershaw, R.A. et al. Notch signaling through Jagged-1 is necessary to initiate chondrogenesis in human bone marrow stromal cells but must be switched off to complete chondrogenesis. Stem Cells 26, 666–674 (2008).
Acknowledgements
This work was funded by the North West Regional Development Agency; North West Embryonic Stem Cell Centre is also supported by the UK Medical Research Council and the UK National Institute for Health Research Biomedical Research Funding Scheme. We thank N. Hanley (University of Manchester) for the gift of human fetal cDNA.
Author information
Authors and Affiliations
Contributions
R.A.O., D.R.B., T.E.H. and S.J.K. were responsible for study concept and design, analysis and interpretation of data and preparation of the manuscript. R.A.O., M.A.B., E.T.L., N.B., F.S. and L.M.G. were responsible for the acquisition of data.
Corresponding author
Ethics declarations
Competing interests
R.A.O., D.R.B., T.E.H. and S.J.K. are named inventors on UK Intellectual Property Office patent applications GB 1012495.6 (filed 26 July 2010) and GB 1012559.9 (filed 27 July 2010).
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1,2 and Supplementary Figs. 1–8 (PDF 3256 kb)
Rights and permissions
About this article
Cite this article
Oldershaw, R., Baxter, M., Lowe, E. et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28, 1187–1194 (2010). https://doi.org/10.1038/nbt.1683
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1683
This article is cited by
-
Effect of a retinoic acid analogue on BMP-driven pluripotent stem cell chondrogenesis
Scientific Reports (2024)
-
From cells to organs: progress and potential in cartilaginous organoids research
Journal of Translational Medicine (2023)
-
Insights into the present and future of cartilage regeneration and joint repair
Cell Regeneration (2022)
-
Induction of iPSC-derived Prg4-positive cells with characteristics of superficial zone chondrocytes and fibroblast-like synovial cells
BMC Molecular and Cell Biology (2022)
-
Targeting G-quadruplex for rescuing impaired chondrogenesis in WRN-deficient stem cells
Cell & Bioscience (2022)