Abstract
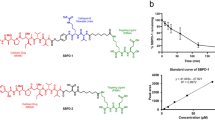
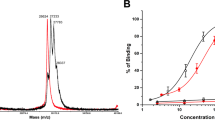
Prostate cancer cells expressing prostate-specific membrane antigen (PSMA) have been targeted with RNA aptamer–small interfering (si)RNA chimeras, but therapeutic efficacy in vivo was demonstrated only with intratumoral injection. Clinical translation of this approach will require chimeras that are effective when administered systemically and are amenable to chemical synthesis. To these ends, we enhanced the silencing activity and specificity of aptamer-siRNA chimeras by incorporating modifications that enable more efficient processing of the siRNA by the cellular machinery. These included adding 2-nucleotide 3′-overhangs and optimizing the thermodynamic profile and structure of the duplex to favor processing of the siRNA guide strand. We also truncated the aptamer portion of the chimeras to facilitate large-scale chemical synthesis. The optimized chimeras resulted in pronounced regression of PSMA-expressing tumors in athymic mice after systemic administration. Anti-tumor activity was further enhanced by appending a polyethylene glycol moiety, which increased the chimeras' circulating half-life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mendenhall, W.M., Henderson, R.H. & Mendenhall, N.P. Definitive radiotherapy for prostate cancer. Am. J. Clin. Oncol. 31, 496–503 (2008).
Potvin, K. & Winquist, E. Hormone-refractory prostate cancer: a primer for the primary care physician. Can. J. Urol. 15 Suppl 1, 14–20 (2008).
Bumcrot, D., Manoharan, M., Koteliansky, V. & Sah, D.W. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2, 711–719 (2006).
Aagaard, L. & Rossi, J.J. RNAi therapeutics: principles, prospects and challenges. Adv. Drug Deliv. Rev. 59, 75–86 (2007).
de Fougerolles, A., Vornlocher, H.P., Maraganore, J. & Lieberman, J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 6, 443–453 (2007).
Ma, J.B., Ye, K. & Patel, D.J. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429, 318–322 (2004).
Schwarz, D.S. et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115, 199–208 (2003).
Khvorova, A., Reynolds, A. & Jayasena, S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell 115, 209–216 (2003).
Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 25, 1149–1157 (2007).
Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 18, 305–319 (2008).
Blow, N. Small RNAs: delivering the future. Nature 450, 1117–1120 (2007).
Kumar, P. et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature 448, 39–43 (2007).
Meade, B.R. & Dowdy, S.F. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv. Drug Deliv. Rev. 59, 134–140 (2007).
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 23, 709–717 (2005).
Peer, D., Zhu, P., Carman, C.V., Lieberman, J. & Shimaoka, M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl. Acad. Sci. USA 104, 4095–4100 (2007).
Rozema, D.B. et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA 104, 12982–12987 (2007).
Hu-Lieskovan, S., Heidel, J.D., Bartlett, D.W., Davis, M.E. & Triche, T.J. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. 65, 8984–8992 (2005).
Heidel, J.D. et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. USA 104, 5715–5721 (2007).
Howard, K.A. et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 14, 476–484 (2006).
Pillé, J.Y. et al. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum. Gene Ther. 17, 1019–1026 (2006).
Reich, S.J. et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 9, 210–216 (2003).
Bitko, V., Musiyenko, A., Shulyayeva, O. & Barik, S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 11, 50–55 (2005).
Li, B.J. et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 11, 944–951 (2005).
DeVincenzo, J. et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral Res. 77, 225–231 (2008).
McNamara, J.O. II et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24, 1005–1015 (2006).
Lupold, S.E., Hicke, B.J., Lin, Y. & Coffey, D.S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 62, 4029–4033 (2002).
Keck, K. et al. Rational design leads to more potent RNA interference against hepatitis B virus: factors effecting silencing efficiency. Mol. Ther. 17, 538–547 (2009).
Czauderna, F. et al. Structural variations and stabilizing modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 31, 2705–2716 (2003).
Boomer, R.M. et al. Conjugation to polyethylene glycol polymer promotes aptamer biodistribution to healthy and inflamed tissues. Oligonucleotides 15, 183–195 (2005).
Rose, S.D. et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 33, 4140–4156 (2005).
Sano, M. et al. Effect of asymmetric terminal structures of short RNA duplexes on the RNA interference activity and strand selection. Nucleic Acids Res. 36, 5812–5821 (2008).
Zhou, J., Li, H., Li, S., Zaia, J. & Rossi, J.J. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol. Ther. 16, 1481–1489 (2008).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 22, 326–330 (2004).
Pall, G.S. & Hamilton, A.J. Improved northern blot method for enhanced detection of small RNA. Nat. Protocols 3, 1077–1084 (2008).
Reagan-Shaw, S. & Ahmad, N. Silencing of polo-like kinase (Plk) 1 via siRNA causes induction of apoptosis and impairment of mitosis machinery in human prostate cancer cells: implications for the treatment of prostate cancer. FASEB J. 19, 611–613 (2005).
Drake, J.M., Gabriel, C.L. & Henry, M.D. Assessing tumor growth and distribution in a model of prostate cancer metastasis using bioluminescence imaging. Clin. Exp. Metastasis 22, 674–684 (2005).
Bozza, M., Sheardy, R.D., Dilone, E., Scypinski, S. & Galazka, M. Characterization of the secondary structure and stability of an RNA aptamer that binds vascular endothelial growth factor. Biochemistry 45, 7639–7643 (2006).
Veronese, F.M. & Mero, A. The impact of PEGylation on biological therapies. BioDrugs 22, 315–329 (2008).
Reagan-Shaw, S. & Ahmad, N. Polo-like kinase (Plk) 1 as a target for prostate cancer management. IUBMB Life 57, 677–682 (2005).
Strebhardt, K. & Ullrich, A. Targeting polo-like kinase 1 for cancer therapy. Nat. Rev. Cancer 6, 321–330 (2006).
Judge, A. & MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 19, 111–124 (2008).
Dyke, C.K. et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 114, 2490–2497 (2006).
Chan, M.Y. et al. Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 117, 2865–2874 (2008).
Katz, B. & Goldbaum, M. Macugen (pegaptanib sodium), a novel ocular therapeutic that targets vascular endothelial growth factor (VEGF). Int. Ophthalmol. Clin. 46, 141–154 (2006).
Gilbert, J.C. et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 116, 2678–2686 (2007).
Girvan, A.C. et al. AGRO100 inhibits activation of nuclear factor-kappaB (NF kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol. Cancer Ther. 5, 1790–1799 (2006).
Soundararajan, S., Chen, W., Spicer, E.K., Courtenay-Luck, N. & Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 68, 2358–2365 (2008).
McNamara, J.O. et al. Multivalent 4-1BB binding aptamers costimulate CD8 T cells and inhibit tumor growth in mice. J. Clin. Invest. 118, 376–386 (2008).
Acknowledgements
We thank M. Henry (Department of Molecular Physiology and Biophysics, University of Iowa) for supplying PC-3 and 22Rv1(1.7) luciferase-positive cells, A. Klingelhutz (Department of Microbiology, University of Iowa) for providing immortalized human fibroblasts, J. Houtman (Department of Immunology, University of Iowa) for help with flow cytometry, and R. Sousa (Department of Biochemistry, University of Texas Health Science Center) for providing a plasmid encoding a mutant T7 RNA polymerase. We are also thankful to M. Behlke for help with the manuscript. J.P.D. is supported by an National Institutes of Health (NIH) graduate student training grant to the Molecular and Cellular Biology Program (University of Iowa), K.W.T. is supported by a Ladies Auxiliary to the Veterans of Foreign Wars postdoctoral fellowship, J.O.M. and A.P.M. are supported by the NIH. This research was supported by an American Cancer Society Institutional Research Grant and a Lymphoma SPORE Developmental Research Award to P.H.G.
Author information
Authors and Affiliations
Contributions
J.P.D., X.-y.L., G.S.T., R.M.W., K.W.T., K.R.S. performed research; D.K.M. provided expertise and analyzed data; A.P.M. provided expertise and useful discussions; J.O.M. designed research, wrote the manuscript and provided useful discussions; P.H.G. designed, coordinated and performed research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Duke University (P.H.G. and J.O.M.) and the University of Iowa (P.H.G., J.O.M. and A.P.M.) have applied for patents based on this technology.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 456 kb)
Rights and permissions
About this article
Cite this article
Dassie, J., Liu, Xy., Thomas, G. et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol 27, 839–846 (2009). https://doi.org/10.1038/nbt.1560
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1560
This article is cited by
-
Structural optimization of siRNA conjugates for albumin binding achieves effective MCL1-directed cancer therapy
Nature Communications (2024)
-
ΔPSap4#5 surface-functionalized abiraterone-loaded nanoparticle successfully inhibits carcinogen-induced prostate cancer in mice: a mechanistic investigation
Cancer Nanotechnology (2023)
-
Advances in PSMA-targeted therapy for prostate cancer
Prostate Cancer and Prostatic Diseases (2022)
-
Targeting signaling pathways in prostate cancer: mechanisms and clinical trials
Signal Transduction and Targeted Therapy (2022)
-
Albumin-Binding Aptamer Chimeras for Improved siRNA Bioavailability
Cellular and Molecular Bioengineering (2022)