Abstract
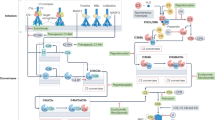
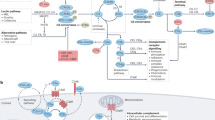
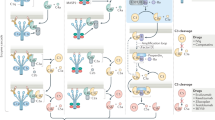
The complement system is a central component of innate immunity and bridges the innate to the adaptive immune response. However, it can also turn its destructive capabilities against host cells and is involved in numerous diseases and pathological conditions. Modulation of the complement system has been recognized as a promising strategy in drug discovery, and a large number of therapeutic modalities have been developed. However, successful marketing of complement-targeted drugs has proved to be more difficult than initially expected, and many strategies have been discontinued. The US Food and Drug Administration's approval of the first complement-specific drug, an antibody against complement component C5 (eculizumab; Soliris), in March 2007, was a long-awaited breakthrough in the field. Approval of eculizumab validates the complement system as therapeutic target and might facilitate clinical development of other promising drug candidates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Katie Ris-Vicari


Similar content being viewed by others
References
Nuttall, G. Experimente über die bacterienfeindlichen Einflüsse des thierischen Körpers. Z. Hyg. Infektionskr. 4, 353–394 (1888).
Ehrlich, P. & Morgenroth, J. Ueber haemolysine—zweite mittheilung. Berl. Klin. Wochenschr., 481–486 (1899).
US Food and Drug Administration. FDA approves first-of-its-kind drug to treat rare blood disorder (USFDA, Rockville, MD, USA) (http://www.fda.gov/bbs/topics/NEWS/2007/NEW01589.html) (March 16, 2007).
Sunyer, J.O., Zarkadis, I.K. & Lambris, J.D. Complement diversity: a mechanism for generating immune diversity? Immunol. Today 19, 519–523 (1998).
Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Walport, M.J. Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001).
Longhi, M.P., Harris, C.L., Morgan, B.P. & Gallimore, A. Holding T cells in check–a new role for complement regulators? Trends Immunol. 27, 102–108 (2006).
Morgan, B.P., Marchbank, K.J., Longhi, M.P., Harris, C.L. & Gallimore, A.M. Complement: central to innate immunity and bridging to adaptive responses. Immunol. Lett. 97, 171–179 (2005).
Mastellos, D. & Lambris, J.D. Complement: more than a 'guard' against invading pathogens? Trends Immunol. 23, 485–491 (2002).
Atkinson, J.P. & Frank, M.M. Bypassing complement: evolutionary lessons and future implications. J. Clin. Invest. 116, 1215–1218 (2006).
Markiewski, M.M., Nilsson, B., Nilsson Ekdahl, K., Mollnes, T.E. & Lambris, J.D. Complement and coagulation: strangers or partners in crime? Trends Immunol. 28, 184–192 (2007).
Hourcade, D.E. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J. Biol. Chem. 281, 2128–2132 (2006).
Spitzer, D., Mitchell, L.M., Atkinson, J.P. & Hourcade, D.E. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 179, 2600–2608 (2007).
Kirkitadze, M.D. & Barlow, P.N. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol. Rev. 180, 146–161 (2001).
Soares, D.C. & Barlow, P.N. Complement control protein modules in the regulators of complement activation. in Structural Biology of the Complement System. (eds. Morikis, D. & Lambris, J.D.) 19–62 (CRC Press, Boca Raton, Florida, 2005).
Volanakis, J.E. & Frank, M. (eds.). The Human Complement System in Health and Disease. (Marcel Dekker, Inc., New York, 1998).
Sahu, A. & Lambris, J.D. Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics. Immunopharmacology 49, 133–148 (2000).
Markiewski, M.M. & Lambris, J.D. The role of complement in inflammatory diseases—from behind the scenes into the spotlight. Am. J. Pathol. 171, 715–727 (2007).
Manderson, A.P., Botto, M. & Walport, M.J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22, 431–456 (2004).
Guo, R.F. & Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23, 821–852 (2005).
Bonifati, D.M. & Kishore, U. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 44, 999–1010 (2007).
Zipfel, P.F., Heinen, S., Jozsi, M. & Skerka, C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43, 97–106 (2006).
Davis, A.E. III., The pathophysiology of hereditary angioedema. Clin. Immunol. 114, 3–9 (2005).
Wagenaar-Bos, I.G. & Hack, C.E. Structure and function of C1-inhibitor. Immunol. Allergy Clin. North Am. 26, 615–632 (2006).
Hill, A., Richards, S.J. & Hillmen, P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 137, 181–192 (2007).
Rooijakkers, S.H. & van Strijp, J.A. Bacterial complement evasion. Mol. Immunol. 44, 23–32 (2007).
Datta, P.K. & Rappaport, J. HIV and complement: hijacking an immune defense. Biomed. Pharmacother. 60, 561–568 (2006).
Hammel, M. et al. A structural basis for complement inhibition by Staphylococcus aureus. Nat. Immunol. 8, 430–437 (2007).
de Haas, C.J. et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199, 687–695 (2004).
Rooijakkers, S.H. et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 6, 920–927 (2005).
Bureeva, S., Andia-Pravdivy, J. & Kaplun, A. Drug design using the example of the complement system inhibitors' development. Drug Discov. Today 10, 1535–1542 (2005).
Makrides, S.C. Therapeutic inhibition of the complement system. Pharmacol. Rev. 50, 59–87 (1998).
Lambris, J.D. & Holers, V.M. (eds.) Therapeutic Interventions in the Complement System (Humana Press, Totowa, NJ, USA, 2000).
Holland, M.C., Morikis, D. & Lambris, J.D. Synthetic small-molecule complement inhibitors. Curr. Opin. Investig. Drugs 5, 1164–1173 (2004).
Arkin, M.R. & Wells, J.A. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat. Rev. Drug Discov. 3, 301–317 (2004).
Sim, R.B. & Tsiftsoglou, S.A. Proteases of the complement system. Biochem. Soc. Trans. 32, 21–27 (2004).
Narayana, S.V., Babu, Y.S. & Volanakis, J.E. Inhibition of complement serine proteases as a therapeutic strategy. in Therapeutic Interventions in the Complement System. (eds. J.D. Lambris & V.M. Holers) 57–74 (Humana Press, Totowa, New Jersey, 2000).
Szalai, A.J. et al. The Arthus reaction in rodents: species-specific requirement of complement. J. Immunol. 164, 463–468 (2000).
Buerke, M., Schwertz, H., Seitz, W., Meyer, J. & Darius, H. Novel small molecule inhibitor of C1s exerts cardioprotective effects in ischemia-reperfusion injury in rabbits. J. Immunol. 167, 5375–5380 (2001).
Agostoni, A., Cicardi, M., Bergamaschini, L., Boccassini, G. & Tucci, A. C1-inhibitor concentrate for treatment of hereditary angioedema. N. Engl. J. Med. 303, 527 (1980).
Kirschfink, M. & Mollnes, T.E. C1-inhibitor: an anti-inflammatory reagent with therapeutic potential. Expert Opin. Pharmacother. 2, 1073–1083 (2001).
De Serres, J., Groner, A. & Lindner, J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus. Apher. Sci. 29, 247–254 (2003).
Longhurst, H.J., Carr, S. & Khair, K. C1-inhibitor concentrate home therapy for hereditary angioedema: a viable, effective treatment option. Clin. Exp. Immunol. 147, 11–17 (2007).
Bork, K., Barnstedt, S.E., Koch, P. & Traupe, H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 356, 213–217 (2000).
Pensky, J., Levy, L.R. & Lepow, I.H. Partial purification of a serum inhibitor of C′1-esterase. J. Biol. Chem. 236, 1674–1679 (1961).
Caliezi, C. et al. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol. Rev. 52, 91–112 (2000).
Nielsen, E.W. et al. Effect of supraphysiologic levels of C1-inhibitor on the classical, lectin and alternative pathways of complement. Mol. Immunol. 44, 1819–1826 (2007).
Lauterbach, M. et al. C1-esterase inhibitor reverses functional consequences of superior mesenteric artery ischemia/reperfusion by limiting reperfusion injury and restoring microcirculatory perfusion. Shock 27, 75–83 (2007).
Lev Pharmaceuticals. Lev Pharmaceuticals reports positive results in pivotal phase III trial for hereditary angioedema (Lev Pharmaceuticals, New York) (http://www.levpharma.com/investors.news.3.14.07.aspx) (March 14, 2007).
Zuraw, B.L. Novel therapies for hereditary angioedema. Immunol. Allergy Clin. North Am. 26, 691–708 (2006).
van Doorn, M.B. et al. A phase I study of recombinant human C1 inhibitor in asymptomatic patients with hereditary angioedema. J. Allergy Clin. Immunol. 116, 876–883 (2005).
Brook, E., Herbert, A.P., Jenkins, H.T., Soares, D.C. & Barlow, P.N. Opportunities for new therapies based on the natural regulators of complement activation. Ann. NY Acad. Sci. 1056, 176–188 (2005).
Weisman, H.F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990).
Li, J.S., Jaggers, J. & Anderson, P.A. The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev. Cardiovasc. Ther. 4, 649–654 (2006).
Avant Immunotherapeutics, Inc. Press Release: AVANT Restructures Organization to Focus Resources on Core Programs and Operations (Avant Immunotherapeutics, Needham, MA) http://phx.corporate-ir.net/phoenix.zhtml?c=93243&p=irol-newsArticle&t=Regular&id=985098& (April 16, 2007).
Rittershaus, C.W. et al. Recombinant glycoproteins that inhibit complement activation and also bind the selectin adhesion molecules. J. Biol. Chem. 274, 11237–11244 (1999).
Smith, R.A. Targeting anticomplement agents. Biochem. Soc. Trans. 30, 1037–1041 (2002).
Hill, A. et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood 107, 2131–2137 (2006).
Presta, L.G. Selection, design, and engineering of therapeutic antibodies. J. Allergy Clin. Immunol. 116, 731–736 (2005).
Frei, Y., Lambris, J.D. & Stockinger, B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol. Cell. Probes 1, 141–149 (1987).
Wang, Y., Rollins, S.A., Madri, J.A. & Matis, L.A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. USA 92, 8955–8959 (1995).
Alexion Pharmaceuticals, Inc., FDA approves Alexion's Soliris(TM) for all patients with PNH—first therapy approved for this rare and life-threatening blood disease (Alexion, Cheshire, CT) http://ir.alexionpharm.com/releasedetail.cfm?ReleaseID=234156 (March 16, 2007).
Thomas, T.C. et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol. Immunol. 33, 1389–1401 (1996).
Proctor, L.M., Woodruff, T.M. & Taylor, S.M. Recent developments in C5/C5a inhibitors. Expert Opin. Ther. Pat. 16, 445–458 (2006).
Adis International Ltd. Eculizumab. Drugs R D. 8, 61–68 (2007).
Whiss, P.A. Pexelizumab Alexion. Curr. Opin. Investig. Drugs 3, 870–877 (2002).
Armstrong, P.W. et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. J. Am. Med. Assoc. 297, 43–51 (2007).
Morrow, T. A promising theory stumbles in clinical trials. Manag. Care 16, 69–70 (2007).
Steve Mitchell. Analysis: Alexion's pexelizumab fails (January 2, 2007) http://www.upi.com/Health_Business/Analysis/2007/01/02/analysis_alexions_pexelizumab_fails/6113/
Taube, C. et al. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc. Natl. Acad. Sci. USA 103, 8084–8089 (2006).
Teeling, J.L. et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104, 1793–1800 (2004).
Teeling, J.L. et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 177, 362–371 (2006).
Sahu, A., Kay, B.K. & Lambris, J.D. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 157, 884–891 (1996).
Janssen, B.J., Halff, E.F., Lambris, J.D. & Gros, P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition, J. Biol. Chem. 282, 29241–29247 (2007).
Sahu, A., Morikis, D. & Lambris, J.D. Compstatin, a peptide inhibitor of complement, exhibits species-specific binding to complement component C3. Mol. Immunol. 39, 557–566 (2003).
Katragadda, M., Magotti, P., Sfyroera, G. & Lambris, J.D. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 49, 4616–4622 (2006).
Potentia Pharmaceuticals, Inc., Potentia Pharmaceuticals announces initiation of phase I clinical trials to evaluate its lead compound for age-related macular degeneration (Potentia, Louisville, KY) http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/03-20-2007/0004549227&EDATE= (March 20, 2007).
Biesecker, G., Dihel, L., Enney, K. & Bendele, R.A. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 42, 219–230 (1999).
Bunka, D.H. & Stockley, P.G. Aptamers come of age—at last. Nat. Rev. Microbiol. 4, 588–596 (2006).
Ng, E.W. et al. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 5, 123–132 (2006).
Monk, P.N., Scola, A.M., Madala, P. & Fairlie, D.P. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol., 152, 429–448 (2007).
Chen, N.J. et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446, 203–207 (2007).
Allegretti, M. et al. Targeting C5a: recent advances in drug discovery. Curr. Med. Chem. 12, 217–236 (2005).
Wong, A.K., Taylor, S.M. & Fairlie, D.P. Development of C5a receptor antagonists. IDrugs 2, 686–693 (1999).
Kohl, J. Drug evaluation: the C5a receptor antagonist PMX-53. Curr. Opin. Mol. Ther. 8, 529–538 (2006).
March, D.R. et al. Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity. Mol. Pharmacol. 65, 868–879 (2004).
Woodruff, T.M. et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 20, 1407–1417 (2006).
Petersen, K.A. et al. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J. Clin. Immunol. 26, 465–475 (2006).
Bureeva, S. et al. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg. Med. Chem. 15, 3489–3498 (2007).
Janssen, B.J. et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 437, 505–511 (2005).
Janssen, B.J., Christodoulidou, A., McCarthy, A., Lambris, J.D. & Gros, P. Structure of C3b reveals conformational changes that underlie complement activity. Nature 444, 213–216 (2006).
Wiesmann, C. et al. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 444, 217–220 (2006).
Nagar, B., Jones, R.G., Diefenbach, R.J., Isenman, D.E. & Rini, J.M. X-ray crystal structure of C3d: a C3 fragment and ligand for complement receptor 2. Science 280, 1277–1281 (1998).
Milder, F.J. et al. Factor B structure provides insights into activation of the central protease of the complement system. Nat. Struct. Mol. Biol. 14, 224–228 (2007).
Narayana, S.V. et al. Structure of human factor D. A complement system protein at 2.0 A resolution. J. Mol. Biol. 235, 695–708 (1994).
Katschke, K.J. Jr. et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J. Exp. Med. 204, 1319–1325 (2007).
Keller, T.H., Pichota, A. & Yin, Z. A practical view of 'druggability'. Curr. Opin. Chem. Biol. 10, 357–361 (2006).
Lipinski, C.A., Lombardo, F., Dominy, B.W. & Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Hopkins, A.L. & Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Gould Rothberg, B.E., Pena, C.E.A. & Rothberg, J.M. A systems biology approach to target identification and validation for human chronic disease drug discovery. Modern Biopharmaceuticals, vol. 1 (ed. Knäblein, J.) 99–125 (Wiley-VCH Verlag, 2005).
Adams, C.P. & Brantner, V.V. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff. (Millwood) 25, 420–428 (2006).
Haffner, M.E., Whitley, J. & Moses, M. Two decades of orphan product development. Nat. Rev. Drug Discov. 1, 821–825 (2002).
Title 21. United States Code (USC) Section 360ee.
Acknowledgements
We thank Peter Ward, Wenchao Song and Maciej Markiewski for their input and for critically reading the manuscript and Deborah McClellan for editorial assistance. This work was supported by National Institutes of Health grants GM-069736, GM-62134, AI-30040, EB003968, CA112162 and AI-068730.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.D.L., along with the University of Pennsylvania, has one issued and five pending patents on compstatin. These patents have been licensed for ophthalmic indications to Potentia Pharmaceuticals, Inc. J.D.L. is also a nonequity-holding member of the Scientific Advisory Board of Potentia Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Ricklin, D., Lambris, J. Complement-targeted therapeutics. Nat Biotechnol 25, 1265–1275 (2007). https://doi.org/10.1038/nbt1342
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1342
This article is cited by
-
The complement factor H-related protein-5 (CFHR5) exacerbates pathological bone formation in ankylosing spondylitis
Journal of Molecular Medicine (2024)
-
Development and classification of RNA aptamers for therapeutic purposes: an updated review with emphasis on cancer
Molecular and Cellular Biochemistry (2023)
-
Identification of pan-kinase-family inhibitors using graph convolutional networks to reveal family-sensitive pre-moieties
BMC Bioinformatics (2022)
-
Neural transcriptomic signature of chronic wasting disease in white-tailed deer
BMC Genomics (2022)
-
Emerging Approaches for the Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN): Therapeutic Potential of the C5a/C5aR Axis
Pain and Therapy (2022)