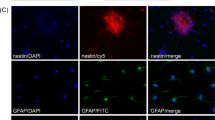
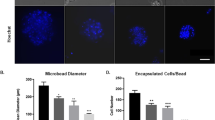
Abstract
Hypoxic-ischemic injury is a prototype for insults characterized by extensive tissue loss. Seeding neural stem cells (NSCs) onto a polymer scaffold that was subsequently implanted into the infarction cavities of mouse brains injured by hypoxia-ischemia allowed us to observe the multiple reciprocal interactions that spontaneously ensue between NSCs and the extensively damaged brain: parenchymal loss was dramatically reduced, an intricate meshwork of many highly arborized neurites of both host- and donor-derived neurons emerged, and some anatomical connections appeared to be reconstituted. The NSC–scaffold complex altered the trajectory and complexity of host cortical neurites. Reciprocally, donor-derived neurons were seemingly capable of directed, target-appropriate neurite outgrowth (extending axons to the opposite hemisphere) without specific external instruction, induction, or genetic manipulation of host brain or donor cells. These “biobridges” appeared to unveil or augment a constitutive reparative response by facilitating a series of reciprocal interactions between NSC and host, including promoting neuronal differentiation, enhancing the elaboration of neural processes, fostering the re-formation of cortical tissue, and promoting connectivity. Inflammation and scarring were also reduced, facilitating reconstitution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davenport, R. & Dennis, M. Neurological emergencies: acute stroke. J. Neurol. Neurosurg. & Psychiatry. 68, 277–288 (2000).
Vannucci, R.C. & Perlman, J.M. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatr. 100, 1004–1014 (1997).
du Plessis, A.J. & Johnston, M.V. Hypoxic-ischemic brain injury in the newborn. Cellular mechanisms and potential strategies for neuroprotection. Clin. Perinatol. 24, 627–654 (1997).
del Zoppo, G. et al. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 10, 95–112 (2000).
Tan, S. & Parks, D.A. Preserving brain function during neonatal asphyxia. Clin. Perinatol. 26, 733–747 (1999).
Sharp, F.R. Transplant for stroke patients? Ann. Neurol. 34, 322–323 (1995).
Ourednik, V. et al. Segregation of human neural stem cells in the developing primate forebrain. Science 293, 1820–1824 (2001).
Snyder, E.Y., Taylor, R.M. & Wolfe, J.H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature 374, 367–370 (1995).
Martinez-Serrano, A. & Björklund, A. Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J. Neurosci. 16, 4604–4616 (1996).
Snyder, E.Y., Yoon, C., Flax, J.D. & Macklis, J.D. Multipotent neural precursors can differentiate toward replacements of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl. Acad. Sci. USA 94, 11663–11668 (1997).
Rosario, C.M. et al. Differentiation of engrafted multipotent neural progenitors towards replacement of missing granule neurons in Meander tail cerebellum may help determine the locus of mutant gene action. Development 124, 4213–4224 (1997).
Gage, F.H. Cell therapy. Nature 392 (Suppl.), 18–24 (1998).
Akerud, P., Canals, J.M., Snyder, E.Y. & Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J. Neurosci. 21, 8108–8118 (2001).
Park, K.I. et al. Transplantation of neural progenitor and stem cells: developmental insights may suggest new therapies for spinal cord and other CNS dysfunction. J. Neurotrauma 16, 675–687 (1999).
Snyder, E.Y. & Park, K.I. Limitations in brain repair. Nat. Med. 8, 928–930 (2002).
Hodges, H. et al. Conditionally immortal neuroepithelial stem cell grafts reverse age-associated memory impairments in rats. Neuroscience 101, 945–955 (2000).
Langer, R. & Vacanti, J.P. Tissue engineering. Science 260, 920–926 (1993).
Colton, C.K. Implantable biohybrid artificial organs. Cell Transplant. 4, 415–436 (1995).
Shalaby, S.W. & Johnson, R.A. Synthetic absorbable polyesters. in Biomedical Polymers (ed. Shalaby, S.W.) 2–34 (Carl Hanser Verlag, München, 1994).
Kim, B.S. & Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 16, 224–230 (1998).
Nerem, R.M. & Sambanis, A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1, 3–13 (1995).
Putnam, A.J. & Mooney, D.J. Tissue engineering using synthetic extracellular matrices. Nat. Med. 2, 824–826 (1996).
Vacanti, J.P. & Langer, R.S. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354 (Suppl.) 1, S32–S34 (1999).
Oberpenning, F., Meng, J., Yoo, J.J. & Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 17, 149–155 (1999).
Mooney, D.J. et al. Stabilized polyglycolic acid fiber-based tubes for tissue engineering. Biomaterials 17, 115–124 (1996).
Puelacher, W.C. et al. Design of nasoseptal cartilage replacements synthesized from biodegradable polymers and chondrocytes. Biomaterials 15, 774–776 (1994).
Kim, W.S. et al. Cartilage configuration in predetermined shapes employing cell transplantation on prosthetic biodegradable synthetic polymers. Plas. Reconstr. Surg. 94, 233–237 (1994).
Vannucci, R.C. Experimental models of perinatal hypoxic-ischemic brain damage. APMIS Suppl. 40, 89–95 (1993).
Veenman, C.L., Reiner, A. & Honig, M.G. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J. Neurosci. Methods 41, 239–254 (1992).
Rajakumar, N., Elisevich, K. & Flumerfelt, B.A. Biotinylated dextran: a versatile anterograde and retrograde neuronal tracer. Brain Res. 607, 47–53 (1993).
Rakic, P. & Caviness, V.S. Jr., Cortical development: view from neurological mutants two decades later. Neuron 14, 1101–1104 (1995).
Deuel, T.F. Growth factors. in Principles of Tissue Engineering (eds Lanza, R.P., Langer, R. & Chick, W.L.) 133–150 (Academic Press, San Diego, CA, 1997).
Labhasetwar, V., Bonadio, J., Goldstein, S., Chen, W. & Levy, R.J. A DNA controlled-release coating for gene transfer: transfer in skeletal and cardiac muscle. J. Pharm. Sci. 87, 1347–1350 (1998).
Shea, L.D., Smiley, E., Bonadio, J. & Mooney, D.J. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 17, 551–554 (1999).
Teng, Y.D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 99, 3024–3029 (2002).
Acknowledgements
This work was supported in part by grants to K.I.P. from the Stem Cell Research Program of the Korean Ministry of Science and Technology and CMB-Yuhan grant of Yonsei University College of Medicine Research Fund of 1998 and the Basic Research program of the Korean Science and Engineering Foundation, and by grants to E.Y.S. from the March of Dimes, National Institutes of Neurological Diseases & Stroke, and Project ALS. We thank Erin Lavik and Robert Langer for the scanning electron micrograph in Figure 2A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Park, K., Teng, Y. & Snyder, E. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol 20, 1111–1117 (2002). https://doi.org/10.1038/nbt751
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt751
This article is cited by
-
Neurogenic Cell Behavior in 3D Culture Enhanced Within a Highly Compliant Synthetic Hydrogel Platform Formed via Competitive Crosslinking
Cellular and Molecular Bioengineering (2024)
-
BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification
Stem Cell Research & Therapy (2019)
-
Safety and efficacy evaluations of an adeno-associated virus variant for preparing IL10-secreting human neural stem cell-based therapeutics
Gene Therapy (2019)
-
Neural stem cell therapy for subacute and chronic ischemic stroke
Stem Cell Research & Therapy (2018)
-
Brain and spinal cord injury repair by implantation of human neural progenitor cells seeded onto polymer scaffolds
Experimental & Molecular Medicine (2018)