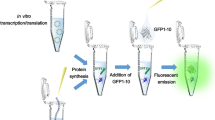
Abstract
Formation of the chromophore of green fluorescent protein (GFP) depends on the correct folding of the protein. We constructed a "folding reporter" vector, in which a test protein is expressed as an N-terminal fusion with GFP. Using a test panel of 20 proteins, we demonstrated that the fluorescence of Escherichia coli cells expressing such GFP fusions is related to the productive folding of the upstream protein domains expressed alone. We used this fluorescent indicator of protein folding to evolve proteins that are normally prone to aggregation during expression in E. coli into closely related proteins that fold robustly and are fully soluble and functional. This approach to improving protein folding does not require functional assays for the protein of interest and provides a simple route to improving protein folding and expression by directed evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
King, J., Haasepettingell, C., Robinson, A.S., Speed, M. & Mitraki, A. Thermolabile folding intermediates: inclusion-body precursors and chaperonin substrates. FASEB J. 10, 57–66 (1996).
Makrides, S.C. Strategies for achieving high-level expression of genes in Escherichia-coli . Microbiol. Rev. 60, 512– 538 (1996).
Plaxco, K.W., Simons, K.T. & Baker, D. Contact order, transition-state placement and the refolding rates of single-domain proteins. J. Mol. Biol. 277, 985–994 (1998).
Wilkinson D.L. & Harrison R.G. Predicting the solubility of recombinant proteins in Escherichia-coli. Bio/Technology 9, 443–448 (1991).
Anfinsen, C.B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Stemmer, W.P.C. Rapid evolution of a protein in-vitro by DNA shuffling. Nature 370, 389–391 ( 1994).
Macbeath, G., Kast, P. & Hilvert, D. Redesigning enzyme topology by directed evolution. Science 279, 1958–1961 ( 1998).
Martineau, P., Jones, P. & Winter, G. Expression of an antibody fragment at high-levels in the bacterial cytoplasm. J. Mol. Biol. 280, 117–127 (1998).
Proba, K., Worn, A., Honegger, A. & Pluckthun, A. Antibody scFv fragments without disulfide bonds made by molecular evolution. J. Mol. Biol. 275, 245–253 (1998).
Ward, W.W. in Bioluminescence and chemiluminescence (eds DeLuca, M.A. & McElroy, W.D.) 235–242 (Academic, New York; 1981).
Cody, C.W., Prasher, D.C., Westler, W.M., Prendergast, F.G. & Ward, W.W. Chemical-structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry 32, 1212–1218 (1993).
Fitz-Gibbon, S. et al. A fosmid-based genomic map and identification of 474 genes of the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles 1, 36–51 ( 1997).
Zubay, G. In vitro synthesis of protein in microbial systems. Annu. Rev. Genet. 7, 267–287 ( 1973).
Neidhardt, F.C. in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology (ed. Neidhardt, F.C.) 3– 6 (American Society of Microbiology, Washington, DC; 1987).
Fedorov, A.N. & Baldwin, T.O. Cotranslational protein-folding. J. Biol. Chem. 272, 32715– 32718 (1997).
Arnold, F.H. Directed evolution: creating biocatalysts for the future. Chem. Eng. Sci. 51, 5091–5102 (1996).
Zhao, H.M. & Arnold, F.H. Optimization of DNA shuffling for high-fidelity recombination. Nucleic Acids Res. 25, 1307–1308 (1997).
Terwilliger, T.C., Zabin, H.B., Horvath, M.P., Sandberg, W.S. & Schlunk, P.M. In-vivo characterization of mutants of the bacteriophage-F1 gene-V protein isolated by saturation mutagenesis. J. Mol. Biol. 236, 556– 571 (1994).
Dickey, L.F. et al. Differences in the regulation of messenger-RNA for housekeeping and specialized-cell ferritin: a comparison of 3 distinct ferritin complementary DNAS, the corresponding subunits, and identification of the 1st processed pseudogene in Amphibia. J. Biol. Chem. 262, 7901–7907 (1987).
Waldo, G.S. & Theil, E.C. in Ferritin and Iron Biomineralization. Comprehensive Supramolecular Chemistry 5. (vol. ed. Susslick, K.) 65–91 (Pergamon Press, Elsevier Science Ltd, Oxford, UK,1996).
Harrison, P.M. & Arosio P. The ferritins: molecular-properties, iron storage function and cellular-regulation. Biochim. Biophys. Acta-Bioenerg. 1275, 161–203 (1996).
Crameri, A., Whitehorn, E.A., Tate, E. & Stemmer, W.P.C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol. 14, 315– 319 (1996).
Heim, R., Prasher, D.C. & Tsien, R.Y. Wavelength mutations and posttranslational autooxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA 91, 12501–12504 (1994).
Reid, B.G. & Flynn, G.C. Chromophore formation in green fluorescent protein. Biochemistry 36, 6786– 6791 (1997).
Makino, Y., Amada, K., Taguchi, H. & Yoshida, M. Chaperonin-mediated folding of green fluorescent protein. J. Biol. Chem. 272, 12468–12474 (1997).
Jappelli, R., Luzzago, A., Tataseo, P., Pernice, I. & Cesareni G. Loop mutations can cause a substantial conformational change in the carboxy terminus of the ferritin protein. J. Mol. Biol. 227, 532–543 ( 1992).
Cormack, B.P., Valdivia, R.H. & Falkow, S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38 (1996).
Terwilliger, T.C. et al. Class-directed structure determination: foundation for a protein structure initiative. Protein Sci. 9, 1851 –1856 (1998).
Heim R., Cubitt A.B. & Tsien R.Y. Improved green fluorescence. Nature 373, 663–664 (1995).
Zhang, Y. et al. Expression of eukaryotic proteins in soluble form in Escherichia coli. Protein Expr. Purif. 12, 159– 165 (1998).
http://rsb.info.nih.gov/nih-image/. NIH-Image is a public domain image processing program developed at the U.S. by the National Institutes of Health.
Moos, T. & Mollgard, K. A sensitive post-DAB enhancement technique for demonstration of iron in the central-nervous-system. Histochem. J. 99, 471– 475 (1993).
Acknowledgements
We wish to thank Sorel Fitz-Gibbon and Jeffrey Miller (UCLA) for P. aerophilum DNA, sequence data, and BLAST searches. We also thank Thomas Peat, James Jett, Scott Peterson, Chia-Hwa Chang, and Raymond Nanni for helpful discussions and review of the manuscript, and the NIH and LDRD program LANL for generous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldo, G., Standish, B., Berendzen, J. et al. Rapid protein-folding assay using green fluorescent protein. Nat Biotechnol 17, 691–695 (1999). https://doi.org/10.1038/10904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/10904
This article is cited by
-
Discovery and multimerization of cross-reactive single-domain antibodies against SARS-like viruses to enhance potency and address emerging SARS-CoV-2 variants
Scientific Reports (2023)
-
Engineering the xylose metabolism in Schizochytrium sp. to improve the utilization of lignocellulose
Biotechnology for Biofuels and Bioproducts (2022)
-
Probing TDP-43 condensation using an in silico designed aptamer
Nature Communications (2022)
-
Proteome-wide landscape of solubility limits in a bacterial cell
Scientific Reports (2022)
-
DnaK response to expression of protein mutants is dependent on translation rate and stability
Communications Biology (2022)