Abstract
Germinal centres (GCs) promote humoral immunity and vaccine efficacy. In GCs, antigen-activated B cells proliferate, express high-affinity antibodies, promote antibody class switching, and yield B cell memory1,2. Whereas the cytokine milieu has long been known to regulate effector functions that include the choice of immunoglobulin class3,4, both cell-autonomous5 and extrinsic6,7 metabolic programming have emerged as modulators of T-cell-mediated immunity8. Here we show in mice that GC light zones are hypoxic, and that low oxygen tension ( ) alters B cell physiology and function. In addition to reduced proliferation and increased B cell death, low
) alters B cell physiology and function. In addition to reduced proliferation and increased B cell death, low  impairs antibody class switching to the pro-inflammatory IgG2c antibody isotype by limiting the expression of activation-induced cytosine deaminase (AID). Hypoxia induces HIF transcription factors by restricting the activity of prolyl hydroxyl dioxygenase enzymes, which hydroxylate HIF-1α and HIF-2α to destabilize HIF by binding the von Hippel–Landau tumour suppressor protein (pVHL)7. B-cell-specific depletion of pVHL leads to constitutive HIF stabilization, decreases antigen-specific GC B cells and undermines the generation of high-affinity IgG, switching to IgG2c, early memory B cells, and recall antibody responses. HIF induction can reprogram metabolic and growth factor gene expression. Sustained hypoxia or HIF induction by pVHL deficiency inhibits mTOR complex 1 (mTORC1) activity in B lymphoblasts, and mTORC1-haploinsufficient B cells have reduced clonal expansion, AID expression, and capacities to yield IgG2c and high-affinity antibodies. Thus, the normal physiology of GCs involves regional variegation of hypoxia, and HIF-dependent oxygen sensing regulates vital functions of B cells. We propose that the restriction of oxygen in lymphoid organs, which can be altered in pathophysiological states, modulates humoral immunity.
impairs antibody class switching to the pro-inflammatory IgG2c antibody isotype by limiting the expression of activation-induced cytosine deaminase (AID). Hypoxia induces HIF transcription factors by restricting the activity of prolyl hydroxyl dioxygenase enzymes, which hydroxylate HIF-1α and HIF-2α to destabilize HIF by binding the von Hippel–Landau tumour suppressor protein (pVHL)7. B-cell-specific depletion of pVHL leads to constitutive HIF stabilization, decreases antigen-specific GC B cells and undermines the generation of high-affinity IgG, switching to IgG2c, early memory B cells, and recall antibody responses. HIF induction can reprogram metabolic and growth factor gene expression. Sustained hypoxia or HIF induction by pVHL deficiency inhibits mTOR complex 1 (mTORC1) activity in B lymphoblasts, and mTORC1-haploinsufficient B cells have reduced clonal expansion, AID expression, and capacities to yield IgG2c and high-affinity antibodies. Thus, the normal physiology of GCs involves regional variegation of hypoxia, and HIF-dependent oxygen sensing regulates vital functions of B cells. We propose that the restriction of oxygen in lymphoid organs, which can be altered in pathophysiological states, modulates humoral immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012)
Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 247, 52–63 (2012)
Xu, Z., Zan, H., Pone, E. J., Mai, T. & Casali, P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat. Rev. Immunol. 12, 517–531 (2012)
Stavnezer, J., Guikema, J. E. & Schrader, C. E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26, 261–292 (2008)
Cui, G. et al. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161, 750–761 (2015)
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998)
Kaelin, W. G. Jr. The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008)
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013)
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014)
Patten, D. A. et al. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol. Biol. Cell 21, 3247–3257 (2010)
Koch, C. J. Importance of antibody concentration in the assessment of cellular hypoxia by flow cytometry: EF5 and pimonidazole. Radiat. Res. 169, 677–688 (2008)
Colgan, S. P. & Taylor, C. T. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 (2010)
Rush, J. S., Liu, M., Odegard, V. H., Unniraman, S. & Schatz, D. G. Expression of activation-induced cytidine deaminase is regulated by cell division, providing a mechanistic basis for division-linked class switch recombination. Proc. Natl Acad. Sci. USA 102, 13242–13247 (2005)
Peng, S. L., Szabo, S. J. & Glimcher, L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl Acad. Sci. USA 99, 5545–5550 (2002)
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008)
Bannard, O. et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity 39, 912–924 (2013)
Wouters, B. G. & Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 (2008)
Krymskaya, V. P. et al. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 25, 1922–1933 (2011)
Keating, R. et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat. Immunol. 14, 1266–1276 (2013)
Zhang, S. et al. B cell-specific deficiencies in mTOR limit humoral immune responses. J. Immunol. 191, 1692–1703 (2013)
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012)
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005)
O’Keeffe, S. et al. Immunoglobulin G subclasses and spirometry in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 4, 932–936 (1991)
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996)
Hatfield, S. M. et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 7, 277ra30 (2015)
Konisti, S., Kiriakidis, S. & Paleolog, E. M. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat. Rev. Rheumatol. 8, 153–162 (2012)
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011)
Karhausen, J. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004)
Liu, Q., Davidoff, O., Niss, K. & Haase, V. H. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J. Clin. Invest. 122, 4635–4644 (2012)
Guertin, D. A. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 (2006)
Woods, M. L., Koch, C. J. & Lord, E. M. Detection of individual hypoxic cells in multicellular spheroids by flow cytometry using the 2-nitroimidazole, EF5, and monoclonal antibodies. Int. J. Radiat. Oncol. Biol. Phys. 34, 93–101 (1996)
Cho, S. H. et al. Glycolytic rate and lymphomagenesis depend on PARP14, an ADP ribosyltransferase of the B aggressive lymphoma (BAL) family. Proc. Natl Acad. Sci. USA 108, 15972–15977 (2011)
Cho, S. H. et al. B cell-intrinsic and -extrinsic regulation of antibody responses by PARP14, an intracellular (ADP-ribosyl)transferase. J. Immunol. 191, 3169–3178 (2013)
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protocols 7, 562–578 (2012)
Eustace, A. et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin. Cancer Res. 19, 4879–4888 (2013)
Acknowledgements
Research funding via National Institutes of Health (NIH) grants R01 AI113292, HL106812 to M.R.B., CA164605 to S.H.; Veterans Affairs Merit award I01 BX002348 to V.H.H., along with support of K.S. by American Cancer Society postdoctoral fellowship PF-13-303-01-DMC, and T.C.B. by T32 DK007563 are gratefully acknowledged, as are O. Davidoff and Q. Liu for expert help with mouse management and with hypoxia chamber usage, respectively, K. Rathmell for critically reading and suggesting manuscript text, P. Young for use of an additional hypoxia chamber, H. Simkins and T. Laufer for guidance on making NP-conjugated fluors, N. Papavasiliou for the AID retrovector, C. Koch for hypoxia detection reagents, and scholarships via the Cancer Center Support Grant (CA068485) and Diabetes Research Center (DK0205930) to help pay for Vanderbilt University cores.
Author information
Authors and Affiliations
Contributions
S.H.C., A.L.R., K.S., E.V., V.H.H., J.W.T. and M.R.B. conceived of and designed experiments; M.R.B. coordinated the research. S.H.C. and M.R.B. analysed all data, and wrote the manuscript, which K.S., J.W.T. and E.V. edited. S.H.C. performed and analysed the immunohistochemistry and flow cytometry for detection of GC hypoxia. S.H.C. and A.L.R. performed and analysed the adoptive transfer and immunization experiments. K.S. and S.H. conceived, performed and analysed RNA-seq experiments. M.W., A.L.R. and S.H.C. performed and analysed class-switching experiments. S.H.C., A.L.R. and T.C.B. performed and analysed the metabolic assays. All other experiments and analyses were performed by S.H.C. and A.L.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks H. Chi and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Landscape of hypoxic cells in follicles and GCs of lymphoid organs.
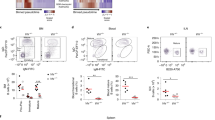
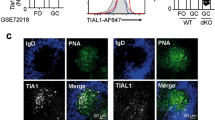
a, b, Controls for anti-HIF-1α antibody staining of GCs and portions of the surrounding splenic follicle, as in Fig. 1a, b, with fluorescent signals at the same intensity settings when analysing samples processed together, using SRBC immunization of wild-type and Hif-deleted mice and either anti-HIF-1α sera or non-immune rabbit IgG (rIgG1), as indicated. Shown are flow cytometry results of intracellular staining performed after exposure of lymphoblasts of the indicated genotypes to 4-hydroxytamoxifen and hypoxia (a), and confocal images (original magnification, ×40) (b), as in Fig. 1a and b, respectively. c, Quantified data obtained from samples represented in Fig. 1c. Shown are the mean (± s.e.m.) specific fluorescence intensities of hypoxyprobe (anti-pimonidazole) staining in GCs (delimited as GL7+) and GL7− IgD+ follicular B cell regions after subtracting background signal (mean fluorescence intensities in these regions after anti-pimonidazole staining of samples from PBS-injected control mice). d, Immunostaining of EF5-modified cells. Shown are confocal microscopic images of spleen sections from SRBC-immunized mice injected with EF5 (left) or PBS (right) 2 h before collection, followed by direct immunofluorescent staining of frozen sections with anti-GL7, anti-IgD and anti-EF5 antibodies, representative of the quantified data presented in Fig. 1d (n = 7 GC from 3 mice in biological replicate analyses). e, Representative images of mesenteric lymph nodes after injections and immunostaining as in Fig. 1c. f, Low magnification (×10; panels are 900 μm × 900 μm) image of anti-pimonidazole immunohistochemistry on spleen sections from SRBC-immunized mice injected with pimonidazole (left) or PBS (right) before collection. Among stained sections for both anti-pimonidazole and EF5, ~75% of GC sections were unequivocally positive (n = 14 sections from 4 spleens in biological replicate analyses). g, Representative images of Peyer’s patches from non-immune, EF5-injected mice processed as in Fig. 1c (n = 6 samples from 3 mice in biological replicate analyses). h, Representative images of spleen sections from unimmunized mice injected with hypoxyprobe (left) or PBS (right) 3 h before collection, processed in parallel with sections from immunized mice injected with probe, and imaged by confocal microscopy at the same time and settings as for the sections from immunized mice (for each, n = 4 sections from 2 spleens in independent biological replicates). i, GSEA plots comparing gene set pre-ranked by log2-fold change in relative expression (GL7+/GL7−) in a hypoxia gene signature.
Extended Data Figure 2 Altered B cell survival, proliferation and metabolism in reduced  .
.
a, Increased executioner caspase-3 activation in hypoxic B cells. Left, representative flow histograms of cleaved caspase-3 (CC3) in the B cell gate after activated B cells were cultured in  of 21% (normoxia) and 1% (hypoxia). B cells were stimulated with BAFF, LPS and IFNγ, cultured for 4 days at the indicated oxygen tension and processed for detection of activated caspase-3 using fluorescent-conjugated CC3 antibody. Right, quantitative data for the frequencies of B cells positive for caspase-3 cleavage in three independent replicate experiments (mean ± s.e.m.). b, O2 sufficiency enhances cell cycle rates. As in a, but cells were pulsed with BrdU and frequencies of S-phase during the cultures are displayed in relation to IgG2c switching. Left, a representative result. Right, quantification of the overall B220+ cell populations in three independent replicate experiments. B cells were cultured for 4 days with BAFF, LPS and IFNγ at the indicated oxygen levels, pulsed for 4 h with BrdU, and then stained with anti-IgG2c, -B220 and -BrdU antibodies after fixation, permeabilization and processing. c, d, Pools of purified wild-type B cells were stimulated with BAFF and LPS, divided and cultured for 2 days in
of 21% (normoxia) and 1% (hypoxia). B cells were stimulated with BAFF, LPS and IFNγ, cultured for 4 days at the indicated oxygen tension and processed for detection of activated caspase-3 using fluorescent-conjugated CC3 antibody. Right, quantitative data for the frequencies of B cells positive for caspase-3 cleavage in three independent replicate experiments (mean ± s.e.m.). b, O2 sufficiency enhances cell cycle rates. As in a, but cells were pulsed with BrdU and frequencies of S-phase during the cultures are displayed in relation to IgG2c switching. Left, a representative result. Right, quantification of the overall B220+ cell populations in three independent replicate experiments. B cells were cultured for 4 days with BAFF, LPS and IFNγ at the indicated oxygen levels, pulsed for 4 h with BrdU, and then stained with anti-IgG2c, -B220 and -BrdU antibodies after fixation, permeabilization and processing. c, d, Pools of purified wild-type B cells were stimulated with BAFF and LPS, divided and cultured for 2 days in  of 21% (normoxia) and 1% (hypoxia). c, Rates of glycolysis were measured after returning to their previous oxygen conditions, using equal numbers of surviving B cells after culture as detailed in the Methods. Glycolysis rates were measured in three independent experiments (mean ± s.e.m.). d, Inhibition of PHD activity decreases cellular respiration of B lymphoblasts. Purified B cells were activated and cultured for 2 days with LPS and BAFF in the presence or absence of DMOG (0.5 mM). The oxygen consumption rate (OCR) was measured with cultured viable B cells (1.5 × 105 cells) (see Methods). The OCR was measured from technical triplicates in one experiment representative of three independent replicates with similar results (mean ± s.d.). e, Metabolic gene expression profile of GL7+ GCB cells. Genes showing significant expression changes in GL7+ GCB cells were mined for genes important for the indicated cellular processes. The heat map depicts values for the indicated genes shown as the value derived as log10 of the fragments per kilobase per million (reads) after adding 1 to each value (FPKM + 1). f, Hypoxia limits switch to IgG among B cells activated via BCRs and CD40. As in Fig. 2a, except that the B cell preparations were activated by cross-linking their surface IgM and CD40 without addition of LPS. g, Quantified mean fluorescence intensities for GFP expression in the full set of replicate experiments conducted as in Fig. 2d, presented as mean (± s.e.m.) data for each condition of culture (
of 21% (normoxia) and 1% (hypoxia). c, Rates of glycolysis were measured after returning to their previous oxygen conditions, using equal numbers of surviving B cells after culture as detailed in the Methods. Glycolysis rates were measured in three independent experiments (mean ± s.e.m.). d, Inhibition of PHD activity decreases cellular respiration of B lymphoblasts. Purified B cells were activated and cultured for 2 days with LPS and BAFF in the presence or absence of DMOG (0.5 mM). The oxygen consumption rate (OCR) was measured with cultured viable B cells (1.5 × 105 cells) (see Methods). The OCR was measured from technical triplicates in one experiment representative of three independent replicates with similar results (mean ± s.d.). e, Metabolic gene expression profile of GL7+ GCB cells. Genes showing significant expression changes in GL7+ GCB cells were mined for genes important for the indicated cellular processes. The heat map depicts values for the indicated genes shown as the value derived as log10 of the fragments per kilobase per million (reads) after adding 1 to each value (FPKM + 1). f, Hypoxia limits switch to IgG among B cells activated via BCRs and CD40. As in Fig. 2a, except that the B cell preparations were activated by cross-linking their surface IgM and CD40 without addition of LPS. g, Quantified mean fluorescence intensities for GFP expression in the full set of replicate experiments conducted as in Fig. 2d, presented as mean (± s.e.m.) data for each condition of culture ( of 21, 5 or 1%, with cytokines and retinoic acid for Ig class switch conditions as indicated, and as for Fig. 2a, b).
of 21, 5 or 1%, with cytokines and retinoic acid for Ig class switch conditions as indicated, and as for Fig. 2a, b).
Extended Data Figure 3 HIF stabilization alters B cell survival, proliferation and class switched antibody level.
a, Purified wild-type B cells were activated and cultured for 4 days with LPS and BAFF in the presence or absence of DMOG, after which frequencies of cells with cleaved caspase 3 or BrdU uptake, as indicated, were measured as in Extended Data Fig. 2 (representative result from one experiment among n = 3 independent replicate experiments). b, Purified wild-type B cells were activated and cultured in conditions for switching to IgG1, IgG2c and IgA, as in Fig. 2a, b, but at atmospheric (21%)  in the presence or absence of DMOG. The frequencies of surface IgG1, IgG2c and IgA among B220+-gated cells were measured as in Fig. 2 (see Methods). FACS plots display the surface levels of IgG1, IgG2c and IgA on B220+-gated cells in one experiment representative of three independent replicates. c, HIF inhibition impedes the hypoxia-induced alteration of antibody class switch choices. B cells were activated and cultured for 4 days with BAFF, LPS and the indicated switching conditions as in Fig. 2a (IL-4, IgG1; IFNγ, IgG2c; retinoic acid, TGFβ, IL-4 and -5, IgA) at
in the presence or absence of DMOG. The frequencies of surface IgG1, IgG2c and IgA among B220+-gated cells were measured as in Fig. 2 (see Methods). FACS plots display the surface levels of IgG1, IgG2c and IgA on B220+-gated cells in one experiment representative of three independent replicates. c, HIF inhibition impedes the hypoxia-induced alteration of antibody class switch choices. B cells were activated and cultured for 4 days with BAFF, LPS and the indicated switching conditions as in Fig. 2a (IL-4, IgG1; IFNγ, IgG2c; retinoic acid, TGFβ, IL-4 and -5, IgA) at  of 21% (normoxia) or 1% (hypoxia) in the presence or absence of the HIF inhibitor Bay 87-2243. FACS plots displaying the surface levels of IgG1, IgG2c and IgA on B220+-gated cells in one representative result among three independent experiments are shown. Flow data shown in this figure were acquired on a BD FACScalibur but otherwise analysed as detailed in the Methods.
of 21% (normoxia) or 1% (hypoxia) in the presence or absence of the HIF inhibitor Bay 87-2243. FACS plots displaying the surface levels of IgG1, IgG2c and IgA on B220+-gated cells in one representative result among three independent experiments are shown. Flow data shown in this figure were acquired on a BD FACScalibur but otherwise analysed as detailed in the Methods.
Extended Data Figure 4 Hypoxia and PHD inhibition repress T-bet induction.
a, b, B cells from wild-type mice were activated and cultured in LPS, BAFF and IL-4 or IFNγ for 4 days under normoxic and hypoxic conditions (a) or cultured with and without DMOG at  of 21% (b). Shown are results of immunoblots using anti-T-bet antibody along with actin as a loading control. Shown is one representative result from three independent experiments. c, HIF-dependent regulation of T-bet expression by pVHL. B cells from wild-type or conditionally deleted VhlΔ/Δ and VhlΔ/Δ HifaΔ/Δ Epas1Δ/Δ (Vhl and V;H1;H2 cKO, respectively) mice were activated and cultured for 4 days in LPS and BAFF in the presence or absence of IFNγ, as indicated. Results of one representative immunoblot (from three independent experiments) probed for HIF-1α, T-bet and actin are shown. d, HIF superinduction by pVHL depletion in B cells at 1%
of 21% (b). Shown are results of immunoblots using anti-T-bet antibody along with actin as a loading control. Shown is one representative result from three independent experiments. c, HIF-dependent regulation of T-bet expression by pVHL. B cells from wild-type or conditionally deleted VhlΔ/Δ and VhlΔ/Δ HifaΔ/Δ Epas1Δ/Δ (Vhl and V;H1;H2 cKO, respectively) mice were activated and cultured for 4 days in LPS and BAFF in the presence or absence of IFNγ, as indicated. Results of one representative immunoblot (from three independent experiments) probed for HIF-1α, T-bet and actin are shown. d, HIF superinduction by pVHL depletion in B cells at 1%  . Wild-type and B cells after conditional Vhlf/f deletion were activated, cultured in 1%
. Wild-type and B cells after conditional Vhlf/f deletion were activated, cultured in 1%  as in Extended Data Fig. 1a, and analysed by flow cytometry after processing together for indirect immunofluorescent staining of intracellular HIF-1α as in Fig. 1a and Extended Data Fig. 1a. Numbers denote the mean fluorescent intensity (MFI) of the B cells of each type. e, Flow cytometric data from one representative experiment as in Fig. 3e, in which B cells were transduced with MIT, MIG, MIT-T-bet or pMx-GFP-AID retrovectors, and cultured with BAFF and LPS ± IFNγ in the presence or absence of DMOG. The frequencies of surface IgG2c+ events among B220+ cells analysed 4 days after transduction are shown, with flow data from one experiment of three independent experiments.
as in Extended Data Fig. 1a, and analysed by flow cytometry after processing together for indirect immunofluorescent staining of intracellular HIF-1α as in Fig. 1a and Extended Data Fig. 1a. Numbers denote the mean fluorescent intensity (MFI) of the B cells of each type. e, Flow cytometric data from one representative experiment as in Fig. 3e, in which B cells were transduced with MIT, MIG, MIT-T-bet or pMx-GFP-AID retrovectors, and cultured with BAFF and LPS ± IFNγ in the presence or absence of DMOG. The frequencies of surface IgG2c+ events among B220+ cells analysed 4 days after transduction are shown, with flow data from one experiment of three independent experiments.
Extended Data Figure 5 VHL regulates antigen-specific antibody production.
a, Schematic outline of adoptive transfer experiments. B cells purified from tamoxifen-treated wild-type, Vhlf/f, or Vhlf/f;Hif1af/f;Epas1f/f;ERT2-Cre mice were transferred into recipients after mixing with CD4+ T cells (polyclonal:OVA-specific OT-II cells = 4:1). Recipients were analysed after primary immunization, or, for memory responses, after the primary and a recall immunization. b, As in Fig. 3a, except B cells from wild-type or conditionally deleted Vhl knockout mice were mixed with CD4+ OT-II TCR transgenic T cells, transferred into Ig CH allotype-disparate (IgHa) recipient mice, followed by immunization with NP-OVA and collected 3 weeks after primary immunization. Donor- (b allotype) and recipient- (a allotype) derived NP-specific IgM and IgG1 levels in the sera were analysed by ELISA. The mean (± s.e.m.) absorbance data averaging independent samples (n = 8 WT and n = 7 Vhl cKO) obtained in two separate transfer experiments (measured on the same ELISA plate) are shown. c, d, As in Fig. 3a, wild-type or VhlΔ/Δ (Vhl cKO) B cells were mixed with wild-type CD4+ T cells (polyclonal:OVA-specific OT-II cells = 4:1), and transferred into Rag0 recipients that were then immunized with NP-OVA, and analysed for NP-specific antibody levels 3 weeks after primary immunization (c) or, for memory response, 9 weeks after the primary immunization and 1 week after a recall immunization (d) (n = 5 independent recipients per genotype in two independent experiments) (c). Mean (± s.e.m.) ELISA data for all-affinity IgM anti-NP from the same samples as Fig. 3b are shown. d, Impaired immune memory follows interference with the B cell hypoxia response system. Terminal sera obtained from the recipient mice (Fig. 3a) 1 week after recall immunization were analysed by ELISA for all-affinity anti-NP antibodies of the indicated isotypes at the same time as the primary response samples (as in c and Fig. 3a).
Extended Data Figure 6 HIF-dependent regulation of antigen-specific B cell population and antibody response by pVHL.
a, b, As in Fig. 3, wild-type, VhlΔ/Δ (Vhl cKO), or VhlΔ/Δ HifaΔ/Δ Epas1Δ/Δ (V;H1;H2 cKO) B cells were mixed with wild-type CD4+ T cells (polyclonal:OVA-specific OT-II cells = 4:1), transferred into Rag0 recipients that were then immunized with NP-OVA and analysed for NP-specific antibody levels after primary immunization as in Fig. 3b, c. Using the same mice and samples as in Fig. 3b, c, cells in spleen secreting IgG2c anti-NP were quantified by ELISpot and averaged as frequencies of antibody-secreting cells (ASC) in the sample (a). Mean (± s.e.m.) frequencies for all samples (n = 9 each) are shown. b, Anti-NP IgA levels in the sera of the samples used in Fig. 3b were quantified by ELISA. c, d, VHL regulation of antigen-specific GCs and memory B cells is HIF-dependent. As in Fig. 3b, c, wild-type, Vhl cKO or V;H1;H2 cKO B cells were mixed with CD4+ T cells (polyclonal:OVA-specific OT-II cells = 4:1), transferred into Rag0 mice, immunized with NP-SRBC along with NP-OVA, boosted with NP-OVA at 3 weeks after primary immunization, and analysed at 1 week after the boost. Shown are the mean (± s.e.m.) frequencies or numbers of antigen (NP)-binding B cells of GC (IgD−GL7+) (c), and early memory (IgD− GL7−CD38hi) phenotypes (d) derived from each donor population and recovered in the recipient mice, as determined by enumeration and flow cytometric phenotyping with fluor-conjugated NP antibody. P values, as indicated in the figure, were derived using Welch’s test for comparisons in a, c and d, where the variances were unequal but followed a normal (Gaussian) distribution.
Extended Data Figure 7 Hypoxia interrupts impairs an activation-induced feed-forward loop in which mTORC1 increases leucine uptake by B cells.
a, PHD inhibition attenuates mTORC1 activity. Wild-type B cells were activated with anti-IgM and cultured for 2 days in BAFF, rested for 20 h in the presence or absence of DMOG, and then re-stimulated for 20 min with anti-IgM. Shown are immunoblots probed with anti-HIF-1α, anti-p-S6K, anti-p-S6 and anti-S6 antibody along with anti-actin as a loading control. Data are the results from one representative experiment among three independent replicates. b–f, Hypoxia and HIF stabilization reduce leucine uptake and mTORC1 activation. b, c, Reduced leucine uptake (b) and Slc7a5 mRNA encoding the large neutral amino acid transporter LAT1 (c) with inhibition of PHD proteins or mTOR. Wild-type cells were analysed after culture in 1% O2 or at  of 21%, in presence of vehicle, DMOG or mTORC1 inhibitor (rapamycin) as indicated. b, B cell uptake of leucine, in n = 3 independent experiments. c, Relative mRNA level, normalized to actin (n = 3 independent experiments). d, e, Activated B cells of the indicated genotypes were assayed for leucine uptake (d) and induction of the Slc7a5 gene encoding a large neutral amino acid transporter (e). d, Leucine uptake by the cultured cells, normalized in each independent experiment (n = 3) to activated wild-type cells. e, VHL loss leads to HIF-dependent attenuation of Slc7a5 mRNA levels. Wild-type or conditional knockout B cells of the indicated genotypes were activated and cultured at 21% O2 as in Fig. 3d. qPCR results normalized first to actin for level within a sample, and then to the wild-type control in each independent experiment (n = 3). f, Leucine stimulates mTORC1 activity in activated B cells. Activated wild-type B cells, divided and cultured overnight in medium lacking or sufficient for the indicated amino acid, were restimulated and analysed as in Fig. 4a, b. Data are mean ± s.e.m.
of 21%, in presence of vehicle, DMOG or mTORC1 inhibitor (rapamycin) as indicated. b, B cell uptake of leucine, in n = 3 independent experiments. c, Relative mRNA level, normalized to actin (n = 3 independent experiments). d, e, Activated B cells of the indicated genotypes were assayed for leucine uptake (d) and induction of the Slc7a5 gene encoding a large neutral amino acid transporter (e). d, Leucine uptake by the cultured cells, normalized in each independent experiment (n = 3) to activated wild-type cells. e, VHL loss leads to HIF-dependent attenuation of Slc7a5 mRNA levels. Wild-type or conditional knockout B cells of the indicated genotypes were activated and cultured at 21% O2 as in Fig. 3d. qPCR results normalized first to actin for level within a sample, and then to the wild-type control in each independent experiment (n = 3). f, Leucine stimulates mTORC1 activity in activated B cells. Activated wild-type B cells, divided and cultured overnight in medium lacking or sufficient for the indicated amino acid, were restimulated and analysed as in Fig. 4a, b. Data are mean ± s.e.m.
Extended Data Figure 8 Hypoxia promotes AMPK activity and induction of the mTORC1 inhibitor REDD1 without repressing mTORC2.
a, B cells were activated and grown for 2 days in LPS and BAFF at the indicated  and in the presence or absence of IFNγ as indicated. ATP concentrations in equal numbers of cells were then assayed. In each of three replicate experiments with similar results, the [ATP] measured for cells at conventional (21%)
and in the presence or absence of IFNγ as indicated. ATP concentrations in equal numbers of cells were then assayed. In each of three replicate experiments with similar results, the [ATP] measured for cells at conventional (21%)  without IFNγ was set as 1, and the mean (± s.e.m.) levels in each sample relative to this reference are shown for three biological replicates. b, Immunoblot results after probing membranes with anti-p-ACC, anti-p-AMPK (T172) and actin are shown for one representative experiment. Numbers indicate the level of signal for cells cultured in hypoxia or DMOG as compared to the reference value of the sample cultured in conventional (21%)
without IFNγ was set as 1, and the mean (± s.e.m.) levels in each sample relative to this reference are shown for three biological replicates. b, Immunoblot results after probing membranes with anti-p-ACC, anti-p-AMPK (T172) and actin are shown for one representative experiment. Numbers indicate the level of signal for cells cultured in hypoxia or DMOG as compared to the reference value of the sample cultured in conventional (21%)  , after normalization of each sample according to its loading. c, Results of a representative qRT–PCR experiment measuring Redd1 mRNA in wild-type B cells (activated and cultured as in b), with each sample first normalized to Actb mRNA and then to vehicle-treated cells. d, e, Effect of VHL, hypoxia and DMOG on Akt phosphorylation in B cells. d, B cells were activated with anti-IgM and BAFF, cultured for 2 days and rested for 20 h under conditions of hypoxia or normoxia in the presence or absence of DMOG, after which cells were re-stimulated (20 min) with anti-ΙgM. e, As in d, B cells from wild-type or conditionally deleted Vhl knockout mice were activated with anti-IgM in the presence of BAFF, cultured for 2 days and rested for 20 h, after which cells were re-stimulated (20 min) with anti-IgM. Shown are results of immunoblots probed with antibodies directed against p-Akt (T308), p-Akt (S473), and Akt. Numbers show the quantification of signal relative to B cells that were not restimulated, after adjustment of each sample for loading as determined by total Akt. Data shown are from one representative experiment among three independent replicates.
, after normalization of each sample according to its loading. c, Results of a representative qRT–PCR experiment measuring Redd1 mRNA in wild-type B cells (activated and cultured as in b), with each sample first normalized to Actb mRNA and then to vehicle-treated cells. d, e, Effect of VHL, hypoxia and DMOG on Akt phosphorylation in B cells. d, B cells were activated with anti-IgM and BAFF, cultured for 2 days and rested for 20 h under conditions of hypoxia or normoxia in the presence or absence of DMOG, after which cells were re-stimulated (20 min) with anti-ΙgM. e, As in d, B cells from wild-type or conditionally deleted Vhl knockout mice were activated with anti-IgM in the presence of BAFF, cultured for 2 days and rested for 20 h, after which cells were re-stimulated (20 min) with anti-IgM. Shown are results of immunoblots probed with antibodies directed against p-Akt (T308), p-Akt (S473), and Akt. Numbers show the quantification of signal relative to B cells that were not restimulated, after adjustment of each sample for loading as determined by total Akt. Data shown are from one representative experiment among three independent replicates.
Extended Data Figure 9 mTORC1 regulates expansion of antigen-specific B cells and antibody class spectrum.
a, Results of immunoblots using anti-raptor and anti-p-S6 antibodies, with anti-S6 antibody as a loading control. B cells (wild-type or haploinsufficient for raptor) were activated with F(ab′)2 anti-IgM and BAFF, cultured for 2 days and rested for 20 h, after which cells were re-stimulated for 20 min with F(ab′)2 anti-IgM. Data shown are from one representative experiment among three independent replicates. b, Recipient antibody controls for effect of mTORC1 on class-switched antibody responses. As in Fig. 4c, wild-type or raptor-haploinsufficent B cells (from heterozygous mice that were Rosa26;ERT2-Cre, Rptorf/+ and converted to Rptor+/Δ by tamoxifen injections) were mixed with CD4+ OT-II TCR transgenic T cells, transferred into Ig CH allotype-disparate recipient mice, immunized with NP-OVA, and obtained 3 weeks after primary immunization. Donor-derived (b allotype) (in Fig. 4) or recipient-derived (a allotype) NP-specific IgG1 and IgG2c levels in the sera were analysed by ELISA. Absorbance data averaging samples (n = 9 WT versus n =8 Rptor+/Δ) obtained in three separate experiments (measured on the same ELISA plate). c–e, Wild-type or Rptor+/Δ B cells were mixed with CD4+ T cells (polyclonal:OVA-specific OT-II = 4:1) and transferred into Rag0 mice and immunized with NP-OVA. Shown are the recoveries of antigen (NP)-binding wild-type versus Rptor+/Δ B cells of GC (B220+ GL7+ IgD−) (c) and early memory (B220+ CD38+ GL7− IgD−) (d) phenotypes. e, Generation of antigen-specific IgG2c-secreting cells depends on mTORC1. Mean (± s.e.m.) results of ELISpot assays quantitating NP-binding IgG2c (b allotype) antibody-secreting cells from the experiments in b and Fig. 4c, d, quantified as described in Extended Data Fig. 6a. P values were derived using Welch’s test for comparisons in c–e, in which the variances were unequal but followed a normal (Gaussian) distribution.
Extended Data Figure 10 mTORC1 is rate-limiting for AID expression and switching to IgG2c.
a, A division-independent mechanism dependent on mTORC1 quantity in B cell switching to IgG2c. Flow cytometric data in the B cell gate, displaying carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) partitioning (fluorescein emission intensities) versus IgG2c, were from one experiment representative of three independent biological replicates. Wild-type or Rptor+/Δ B cells were stained with CFSE and cultured with LPS, BAFF and IFNγ, and analysed by flow cytometry. b, Wild-type or Rptor+/Δ B cells were cultured for 2 days with LPS, BAFF and IFNγ. mRNA levels of the Aidca (left) and Tbx21 (right) genes measured in three independent replicate experiments by qRT–PCR normalized to actin in the sample and then to the level in wild-type cells (set as relative level of 1). c, Immunoblots probed for raptor, T-bet and actin, as indicated, using B cells as in b (representative of n = 3 independent experiments). d, mTOR promotes switching to IgG by division-independent mechanisms. As in a, but CFSE-stained wild-type B cells were activated and cultured for 4 days with LPS, BAFF and IFNγ in the presence or absence of rapamycin versus vehicle. e, f, mTORC1 regulation of AID level in collaboration with T-bet determines efficient switching to IgG2c. B cells were transduced with MIT, MIG, MIT-T-bet or pMx-GFP-AID retrovectors, and cultured with BAFF and LPS and/or IFNγ in the presence or absence of rapamycin (5 nM). e, Representative flow data, from one experiment among three independent replicates, derived as in Extended Data Fig. 4e. f, Frequencies of surface IgG2c+ events among B220+ cells analysed 4 days after transduction are shown (n = 3 independent experiments). Data are mean ± s.e.m.
Supplementary information
Supplementary Figures
This file contains the raw data for Figure 4 and Extended Data Figures 4, 7, 8, 9 and 10. (PDF 5781 kb)
Rights and permissions
About this article
Cite this article
Cho, S., Raybuck, A., Stengel, K. et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 537, 234–238 (2016). https://doi.org/10.1038/nature19334
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19334
This article is cited by
-
MCT1-governed pyruvate metabolism is essential for antibody class-switch recombination through H3K27 acetylation
Nature Communications (2024)
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
B cells ferment sugar before they (affinity) mature
Nature Immunology (2023)
-
Germinal centers FAMished without TFAM
Nature Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.


 .
.