Abstract
Haem is an iron-containing tetrapyrrole that is critical for a variety of cellular and physiological processes1,2,3. Haem binding proteins are present in almost all cellular compartments, but the molecular mechanisms that regulate the transport and use of haem within the cell remain poorly understood2,3. Here we show that haem-responsive gene 9 (HRG-9) (also known as transport and Golgi organization 2 (TANGO2)) is an evolutionarily conserved haem chaperone with a crucial role in trafficking haem out of haem storage or synthesis sites in eukaryotic cells. Loss of Caenorhabditis elegans hrg-9 and its paralogue hrg-10 results in the accumulation of haem in lysosome-related organelles, the haem storage site in worms. Similarly, deletion of the hrg-9 homologue TANGO2 in yeast and mammalian cells induces haem overload in mitochondria, the site of haem synthesis. We demonstrate that TANGO2 binds haem and transfers it from cellular membranes to apo-haemoproteins. Notably, homozygous tango2−/− zebrafish larvae develop pleiotropic symptoms including encephalopathy, cardiac arrhythmia and myopathy, and die during early development. These defects partially resemble the symptoms of human TANGO2-related metabolic encephalopathy and arrhythmias, a hereditary disease caused by mutations in TANGO24,5,6,7,8. Thus, the identification of HRG-9 as an intracellular haem chaperone provides a biological basis for exploring the aetiology and treatment of TANGO2-related disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE198848. Gene information for C. elegans hrg-9 (R186.1) and hrg-10 (Y80D3A.9) are available from Wormbase (https://wormbase.org). Source data are provided with this paper.
References
Severance, S. & Hamza, I. Trafficking of heme and porphyrins in metazoa. Chem. Rev. 109, 4596–4616 (2009).
Reddi, A. R. & Hamza, I. Heme mobilization in animals: a metallolipid’s journey. Acc. Chem. Res. 49, 1104–1110 (2016).
Chambers, I. G., Willoughby, M. M., Hamza, I. & Reddi, A. R. One ring to bring them all and in the darkness bind them: the trafficking of heme without deliverers. Biochim. Biophys. Acta 1868, 118881 (2021).
Kremer, L. S. et al. Bi-allelic truncating mutations in TANGO2 cause infancy-onset recurrent metabolic crises with encephalocardiomyopathy. Am. J. Hum. Genet. 98, 358–362 (2016).
Lalani, S. R. et al. Recurrent muscle weakness with rhabdomyolysis, metabolic crises, and cardiac arrhythmia due to bi-allelic TANGO2 mutations. Am. J. Hum. Genet. 98, 347–357 (2016).
Mingirulli, N. et al. Clinical presentation and proteomic signature of patients with TANGO2 mutations. J. Inherit. Metab. Dis. 43, 297–308 (2020).
Milev, M. P. et al. The phenotype associated with variants in TANGO2 may be explained by a dual role of the protein in ER-to-Golgi transport and at the mitochondria. J. Inherit. Metab. Dis. 44, 426–437 (2021).
Powell, A. R., Ames, E. G., Knierbein, E. N., Hannibal, M. C. & Mackenzie, S. J. Symptom prevalence and genotype–phenotype correlations in patients with TANGO2-related metabolic encephalopathy and arrhythmias (TRMEA). Pediatr. Neurol. 119, 34–39 (2021).
Rao, A. U., Carta, L. K., Lesuisse, E. & Hamza, I. Lack of heme synthesis in a free-living eukaryote. Proc. Natl Acad. Sci. USA 102, 4270–4275 (2005).
Rajagopal, A. et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453, 1127–1131 (2008).
Chen, C., Samuel, T. K., Sinclair, J., Dailey, H. A. & Hamza, I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell 145, 720–731 (2011).
Korolnek, T., Zhang, J., Beardsley, S., Scheffer, G. L. & Hamza, I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 19, 1008–1019 (2014).
Sinclair, J. et al. Inter-organ signalling by HRG-7 promotes systemic haem homeostasis. Nat. Cell Biol. 19, 799–807 (2017).
Bard, F. et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 439, 604–607 (2006).
Grant, B. & Hirsh, D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311–4326 (1999).
Schroeder, L. K. et al. Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Mol. Biol. Cell 18, 995–1008 (2007).
O’Rourke, E. J., Soukas, A. A., Carr, C. E. & Ruvkun, G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 10, 430–435 (2009).
Chen, A. J. et al. Label-free imaging of heme dynamics in living organisms by transient absorption microscopy. Anal. Chem. 90, 3395–3401 (2018).
Hanna, D. A. et al. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl Acad. Sci. USA 113, 7539–7544 (2016).
Ebert, P. S., Hess, R. A., Frykholm, B. C. & Tschudy, D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem. Biophys. Res. Commun. 88, 1382–1390 (1979).
Williams, C. C., Jan, C. H. & Weissman, J. S. Targeting and plasticity of mitochondrial proteins revealed by proximity-specific ribosome profiling. Science 346, 748–751 (2014).
Martinez-Guzman, O. et al. Mitochondrial–nuclear heme trafficking in budding yeast is regulated by GTPases that control mitochondrial dynamics and ER contact sites. J. Cell Sci. 133, jcs237917 (2020).
Yuan, X. et al. Regulation of intracellular heme trafficking revealed by subcellular reporters. Proc. Natl Acad. Sci. USA 113, E5144–E5152 (2016).
Li, Y. et al. MFSD7C switches mitochondrial ATP synthesis to thermogenesis in response to heme. Nat. Commun. 11, 4837 (2020).
Chiabrando, D. et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Invest. 122, 4569–4579 (2012).
Galmozzi, A. et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 576, 138–142 (2019).
Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 11, 739–750 (2010).
Holthuis, J. C. & Menon, A. K. Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48–57 (2014).
Giri, R. P. et al. Continuous uptake or saturation-investigation of concentration and surface-packing-specific hemin interaction with lipid membranes. J. Phys. Chem. B 122, 7547–7554 (2018).
Garber Morales, J. et al. Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast. Biochemistry 49, 5436–5444 (2010).
Ganis, J. J. et al. Zebrafish globin switching occurs in two developmental stages and is controlled by the LCR. Dev. Biol. 366, 185–194 (2012).
Gietz, R. D. & Schiestl, R. H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 7, 253–263 (1991).
Ness, F. et al. Sterol uptake in Saccharomyces cerevisiae heme auxotrophic mutants is affected by ergosterol and oleate but not by palmitoleate or by sterol esterification. J. Bacteriol. 180, 1913–1919 (1998).
Sinclair, P. R., Gorman, N. & Jacobs, J. M. Measurement of heme concentration. Curr. Protoc. Toxicol. 8, Unit 8.3 (2001).
Chen, C. et al. Snx3 regulates recycling of the transferrin receptor and iron assimilation. Cell Metab. 17, 343–352 (2013).
Dickinson, D. J., Ward, J. D., Reiner, D. J. & Goldstein, B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034 (2013).
Kang, J. et al. Caenorhabditis elegans homologue of Fam210 is required for oogenesis and reproduction. J. Genet. Genomics 47, 694–704 (2020).
Arribere, J. A. et al. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198, 837–846 (2014).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
McGhee, J. D. et al. ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev. Biol. 327, 551–565 (2009).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Zhen, R. et al. Wdr26 regulates nuclear condensation in developing erythroblasts. Blood 135, 208–219 (2020).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Fink, M. et al. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 46, 101–113 (2009).
Kawahara, G., Guyon, J. R., Nakamura, Y. & Kunkel, L. M. Zebrafish models for human FKRP muscular dystrophies. Hum. Mol. Genet. 19, 623–633 (2010).
Unissa, A. N., Subbian, S., Hanna, L. E. & Selvakumar, N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. 45, 474–492 (2016).
Unissa, A. N. et al. Significance of catalase-peroxidase (KatG) mutations in mediating isoniazid resistance in clinical strains of Mycobacterium tuberculosis. J. Glob. Antimicrob. Resist. 15, 111–120 (2018).
Hanna, D. A. et al. Heme bioavailability and signaling in response to stress in yeast cells. J. Biol. Chem. 293, 12378–12393 (2018).
Baureder, M., Reimann, R. & Hederstedt, L. Contribution of catalase to hydrogen peroxide resistance in Enterococcus faecalis. FEMS Microbiol. Lett. 331, 160–164 (2012).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Chen, W. et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl Acad. Sci. USA 106, 16263–16268 (2009).
Feissner, R., Xiang, Y. & Kranz, R. G. Chemiluminescent-based methods to detect subpicomole levels of c-type cytochromes. Anal. Biochem. 315, 90–94 (2003).
Piel, R. B. 3rd et al. A novel role for progesterone receptor membrane component 1 (PGRMC1): a partner and regulator of ferrochelatase. Biochemistry 55, 5204–5217 (2016).
Acknowledgements
We thank R. Lill and H. Gao for critical discussions; the Caenorhabditis Genetics Center (CGC), the B. Grant laboratory, and the A. Soukas laboratory for the worm strains used in this study. This work was supported by funding from the National Natural Science Foundation of China (31871200, 31930003 and 32071155 to C.C.), the National Key Research and Development Program of China (2018YFA0507802 to C.C.), the US National Science Foundation (1552791 to A.R.R.), and the US National Institutes of Health (R33ES025661 to A.R.R. and I.H., R35GM145350 to A.R.R., and R01DK074797 to I.H.).
Author information
Authors and Affiliations
Contributions
C.C. conceived the project and wrote the manuscript. F.S. and Y.Z. performed the C. elegans experiments. F.S., J.K. and Y.Z. conducted the RNA-seq experiment. Z.Z. performed the mammalian cell experiments and haem transfer experiments. M.M.W. and A.R.R. designed and executed the yeast experiments. S.S., Z.Z. and M.C. performed zebrafish experiments. Y.S., F.S. and Y.C. performed haem binding experiments. M.C. performed the immunofluorescence experiments. X.Y. and I.H. contributed to the mammalian haem sensor experiments. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Albertha Walhout, Yvette Yien and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Analysis of hrg-9 and hrg-10 expression in C. elegans.
a, Workflow of RNA sequencing experiment. Synchronized L1 worms were grown in mCeHR2 medium containing 2 μM, 20 μM, or 400 μM haem to the gravid stage, and the intestines were isolated for transcriptomics analysis using the SMART-Seq technology. b, The hrg-9p::nls-gfp transcriptional reporter is expressed in the intestine of C. elegans at all developmental stages. nls, nuclear localization signal; i, intestine. Scale bars, 20 μm. c, The hrg-10p::nls-gfp transcriptional reporter is predominantly expressed in the intestine of C. elegans at all developmental stages. nls, nuclear localization signal; i, intestine. Scale bars, 20 μm. d, Knockdown of hrg-9 and hrg-10 by RNAi does not impair the intestinal secretion of YP170::GFP in C. elegans. The known trafficking gene rab-10 is used as a control. i, intestine; o, oocyte; e, embryo. Scale bars, 20 μm. e, RNA-seq demonstrates that intestinal hrg-10 expression is not significantly affected by haem levels in C. elegans. n = 3 independent samples for each group. f, Quantitative RT-PCR shows that haem does not regulate hrg-10 expression at the organismal level in C. elegans. n = 3 independent experiments. g, Haem does not regulate the expression of hrg-10p::nls-gfp transcriptional reporter. Scale bars, 20 μm. Data in e and f are presented as mean ± s.e.m. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.
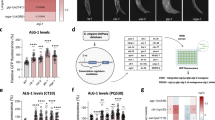
Extended Data Fig. 2 hrg-9 and hrg-10 regulate haem homeostasis in C. elegans.
a, Knockout of hrg-9 and hrg-10 in C. elegans. The sgRNA targeting sites and the positions of genotyping primers (arrows) are shown in the knockout strategies (top), and the genotyping results are shown at the bottom. b, Representative raw mass spectra of 15N-haem and 14N-haem mixture from C. elegans. The 14N-haem shows a mass to charge ratio (m/z) of 679.51 and the 15N-haem shows m/z of 680.51 and 681.56. c, The uptake of 15N-haem in hrg-9 and hrg-10 knockout worms is comparable to that of wild type worms. Worms were incubated with 4 μM 15N-haem for 3 h, and the total haem in worms were extracted for analysis by mass spectrometry. Wild type worms cultured with 200 μM haem were used as a control. n = 3 independent experiments. d,e, Representative images (d) and quantification (e) of zinc mesoporphyrin IX (ZnMP) staining in worms treated with control, mrp-5, hrg-9, or hrg-10 RNAi. The known haem transporter mrp-5 is used as a control. n = 30 worms examined over 3 independent experiments. Scale bars, 20 μm. f, The ZnMP accumulation phenotypes in hrg-9 and hrg-10 knockout worms are rescued by the expression of HRG-9::GFP and HRG-10::GFP, respectively. tg9, HRG-9::GFP; tg10, HRG-10::GFP. n = 30 worms examined over 3 independent experiments. g, Wild type, hrg-9, hrg-10, and hrg-9;hrg-10 adults were stained with ZnMP and LysoSensor Green. Scale bars, 5 μm. h, ZnMP colocalizes with PGP-2::GFP, a marker of lysosome-related organelles, in control, hrg-9, and hrg-10 RNAi worms. Scale bars, 10 μm. Data in c,e,f are presented as mean ± s.e.m. P values were determined by one-way ANOVA followed by Tukey’s test.
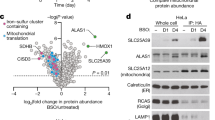
Extended Data Fig. 3 TANGO2 proteins regulate haem homeostasis in yeast and mammalian cells.
a, Growth curve of wild type, hem1Δ, and tango2Δ yeast cells treated with or without 5-aminolevulinic acid (ALA). OD, optical density at 600 nm. n = 2 technical replicates. b, The tango2Δ yeast cells exhibit increased expression (middle) and activity (top) of Ctt1p. GAPDH is used as a control. c,d, Dynamics of haem trafficking to mitochondria (c) and the cytosol (d) in wild type and tango2Δ cells. Labile haem levels in mitochondria and the cytosol were monitored by measuring fractional saturation of the haem sensors specifically targeted to these two locations, respectively, following initiation of haem synthesis. e, Flag-tagged human TANGO2 colocalizes with the mitochondrial marker COX IV in HEK293 cells. Scale bars, 10 μm. f, Western analysis of TANGO2 in whole lysates as well as the mitochondrial and non-mitochondrial fractions of HEK293 cells. The mitochondrial protein COX IV and the cytosolic protein GAPDH were used as controls. g, Strategy to knock out TANGO2 in HEK293 cells and verification of the knockout by genotyping. The sgRNA targeting sites and the positions of genotyping primers are indicated. h, Immunoblotting analysis validating the knockout of TANGO2 in HEK293 cells. i, Total haem content of the wild type and ΔTANGO2 HEK293 cells after treatment with succinylacetone (SA) for 24 h. j, Strategy to knock out Tango2 in MEL cells and verification by PCR genotyping. The sgRNA targeting sites and the positions of genotyping primers are indicated. k, Immunoblotting analysis validating the knockout of Tango2 in MEL cells. l, o-dianisidine staining of haemoglobin in wild type and Tango2-knockout MEL cells. Scale bar, 20 μm. m,n, Basal oxygen consumption rates (OCR, m) and maximal OCR (n) of wild type and TANGO2-knockout HEK293 and MEL cells. o, Representative images of TMRE staining in wild type and TANGO2-knockout HEK293 and MEL cells. Scale bars, 20 μm. n = 3 independent experiments for c,d,i,m,n. Data in a,c,d,i,m,n are presented as mean ± s.e.m. P values were determined by one-way ANOVA followed by Tukey’s test.
Extended Data Fig. 4 Loss of tango2 leads to reduced locomotor activity of zebrafish larvae.
a, Strategy to knock out tango2 in zebrafish and verification of the knockout by PCR genotyping. The sgRNA targeting sites and the positions of PCR primers are indicated. b, Comparison of the sequences between the wild type tango2 and the mutated allele in zebrafish. The exon2 - intron2 junction is deleted in the mutant allele. c, Verification of intron 2 retention in the tango2−/− zebrafish at the mRNA level by reverse transcription (RT)-PCR. d, The predicted protein product of Tango2 in tango2−/− zebrafish. e, Whole-mount in situ hybridization showing maternal expression of zebrafish tango2. Arrowheads indicate the expression of tango2 in 1-cell and 1000-cell stage embryos. Arrows indicate the expression of gata1 (control) in the intermediate cell mass and tango2 in neuronal tissues at 24 h post-fertilization (hpf). Scale bars, 200 μm. f, o-dianisidine staining of wild type and tango2-knockout zebrafish embryos at 48 and 72 hpf. Scale bars, 200 μm. g, Total haem content in wild type and tango2-knockout zebrafish embryos at 48 and 72 hpf. n = 3 independent experiments. Data are presented as mean ± s.e.m. h, Whole-mount in situ hybridization showing normal expression of the erythroid markers gata1 and αe3-globin in tango2-knockout zebrafish embryos. Scale bars, 200 μm. i, Knockout of tango2 did not reduce erythrocytes in Tg(globin-LCR:EGFP) transgenic zebrafish embryos. n = 3 independent experiments. Data are presented as mean ± s.e.m. j, Distance travelled per minute by 12-dpf wild type and tango2-knockout zebrafish larvae during the 10-min dark / 10-min light cycles. Black bars indicate dark and white bars indicate light. n = 16 fish for each group. k, Representative swimming tracks of 12-dpf wild type and tango2-knockout zebrafish larvae over a 40-min period under constant light. Scale bars, 2.5 mm. Statistical significance was determined by one-way ANOVA followed by Tukey’s test.
Extended Data Fig. 5 Knockout of tango2 causes brain and heart defects in zebrafish larvae.
a, Representative micro-CT images of the brains of wild type and tango2-knockout zebrafish larvae at 12 dpf. Images on the left are side views of the 3D reconstructed zebrafish brains. Numbers in the images indicate the distance (μm) from the top of the brain, as shown in the 3D images. Scale bars, 100 μm. b, Quantitative RT-PCR analysis of c-fos in wild type and tango2-knockout zebrafish larvae at 12 dpf. Data are presented as mean ± s.e.m from 3 independent experiments. Each sample contains a pool of 30 zebrafish larvae. c, Heart rates of wild type and tango2-knockout zebrafish larvae at 12 dpf. n = 28 fish for each group. Data are presented as mean ± s.e.m. d, Measurement of systolic time interval and diastolic time interval in wild type and tango2-knockout zebrafish larvae at 7 dpf. n = 12 fish for each group. Data are presented as mean ± s.e.m. e, Detection of heart contractions in 7-dpf wild type and tango2-knockout zebrafish larvae using the Changing Pixel Intensity Algorithm. Peaks indicate individual heart contractions. P values were determined by one-way ANOVA followed by Tukey’s test.
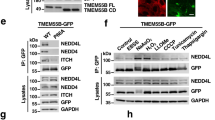
Extended Data Fig. 6 TANGO2 transfers haem in vitro.
a, Quantification of ZnMP fluorescence in wild type, hrg-9 and hrg-10 double knockout worms, and the double knockout worms expressing TANGO2 genes from human (hTANGO2), yeast (ytango2), or zebrafish (ztango2). n = 30 worms examined over 3 independent experiments. Data are presented as mean ± s.e.m. P values were determined by one-way ANOVA followed by Tukey’s test. b,c, Haem transfer from mitochondria isolated from mouse liver to apo-myoglobin in the absence or presence of yeast Tango2p (b) and zebrafish Tango2 (c). d,e, Haem transfer assays for human TANGO2, BSA (d), and GFP (e). Mitochondria isolated from mouse liver were used as the haem donor and apo-myoglobin was used as the haem acceptor. f,g, Incorporation of 10 μM (f) and 1 μM (g) haem into apo-myoglobin in the absence or presence of TANGO2. h, Haem transfer from the mitochondria isolated from TANGO2-knockout HEK293 cells to apo-myoglobin in the absence or presence of TANGO2. i, Coomassie blue staining validating the protein digestion by trypsin in mitochondrial membranes isolated from mouse liver. j, Haem transfer from protein-free mitochondrial membranes to apo-myoglobin in the absence or presence of TANGO2. Mito, mitochondria.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1 and 2.
Supplementary Table 1
List of haem-responsive genes identified by transcriptomics analysis of C. elegans intestines.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, F., Zhao, Z., Willoughby, M.M. et al. HRG-9 homologues regulate haem trafficking from haem-enriched compartments. Nature 610, 768–774 (2022). https://doi.org/10.1038/s41586-022-05347-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05347-z
This article is cited by
-
Mechanisms controlling cellular and systemic iron homeostasis
Nature Reviews Molecular Cell Biology (2024)
-
Thiol-catalyzed formation of NO-ferroheme regulates intravascular NO signaling
Nature Chemical Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.