Abstract
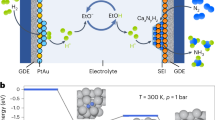
In addition to its use in the fertilizer and chemical industries1, ammonia is currently seen as a potential replacement for carbon-based fuels and as a carrier for worldwide transportation of renewable energy2. Implementation of this vision requires transformation of the existing fossil-fuel-based technology for NH3 production3 to a simpler, scale-flexible technology, such as the electrochemical lithium-mediated nitrogen-reduction reaction3,4. This provides a genuine pathway from N2 to ammonia, but it is currently hampered by limited yield rates and low efficiencies4,5,6,7,8,9,10,11,12. Here we investigate the role of the electrolyte in this reaction and present a high-efficiency, robust process that is enabled by compact ionic layering in the electrode–electrolyte interface region. The interface is generated by a high-concentration imide-based lithium-salt electrolyte, providing stabilized ammonia yield rates of 150 ± 20 nmol s−1 cm−2 and a current-to-ammonia efficiency that is close to 100%. The ionic assembly formed at the electrode surface suppresses the electrolyte decomposition and supports stable N2 reduction. Our study highlights the interrelation between the performance of the lithium-mediated nitrogen-reduction reaction and the physicochemical properties of the electrode–electrolyte interface. We anticipate that these findings will guide the development of a robust, high-performance process for sustainable ammonia production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the paper and its Supplementary Information. Source data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Phil. Trans. R. Soc. B 368, 20130164 (2013).
MacFarlane, D. R. et al. Liquefied sunshine: transforming renewables into fertilizers and energy carriers with electromaterials. Adv. Mater. 32, 1904804 (2020).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
Tsuneto, A., Kudo, A. & Sakata, T. Efficient electrochemical reduction of N2 to NH3 catalyzed by lithium. Chem. Lett. 22, 851–854 (1993).
Tsuneto, A., Kudo, A. & Sakata, T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 367, 183–188 (1994).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Lazouski, N., Chung, M., Williams, K., Gala, M. L. & Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 3, 463–469 (2020).
Andersen, S. Z. et al. Increasing stability, efficiency, and fundamental understanding of lithium-mediated electrochemical nitrogen reduction. Energy Environ. Sci. 13, 4291–4300 (2020).
Cherepanov, P. V., Krebsz, M., Hodgetts, R. Y., Simonov, A. N. & MacFarlane, D. R. Understanding the factors determining the faradaic efficiency and rate of the lithium redox-mediated N2 reduction to ammonia. J. Phys. Chem. C 125, 11402–11410 (2021).
Suryanto, B. H. et al. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science 372, 1187–1191 (2021).
Li, K. et al. Increasing current density of Li-mediated ammonia synthesis with high surface area copper electrodes. ACS Energy Lett. 7, 36–41 (2022).
Li, K. et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 374, 1593–1597 (2021).
Du, H.-L. et al. Is molybdenum disulfide modified with molybdenum metal catalytically active for the nitrogen reduction reaction? J. Electrochem. Soc. 167, 146507 (2020).
Choi, J. et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nat. Commun. 11, 5546 (2020).
Choi, J. et al. Reassessment of the catalytic activity of bismuth for aqueous nitrogen electroreduction. Nat. Catal. 5, 382–384 (2022).
Lazouski, N., Schiffer, Z. J., Williams, K. & Manthiram, K. Understanding continuous lithium-mediated electrochemical nitrogen reduction. Joule 3, 1127–1139 (2019).
Gao, L.-F. et al. Domino effect: gold electrocatalyzing lithium reduction to accelerate nitrogen fixation. Angew. Chem. Int. Ed. 60, 5257–5261 (2021).
Sonoki, H., Matsui, M. & Imanishi, N. Effect of anion species in early stage of SEI formation process. J. Electrochem. Soc. 166, A3593–A3598 (2019).
Shadike, Z. et al. Identification of LiH and nanocrystalline LiF in the solid–electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. 16, 549–554 (2021).
Wang, L., Uosaki, K. & Noguchi, H. Effect of electrolyte concentration on the solvation structure of gold/LiTFSI–DMSO solution interface. J. Phys. Chem. C 124, 12381–12389 (2020).
Zhang, W., Zhuang, H. L., Fan, L., Gao, L. & Lu, Y. A “cation-anion regulation” synergistic anode host for dendrite-free lithium metal batteries. Sci. Adv. 4, eaar4410 (2018).
Tong, J. et al. Insights into the solvation and dynamic behaviors of a lithium salt in organic- and ionic liquid-based electrolytes. Phys. Chem. Chem. Phys. 21, 19216–19225 (2019).
Younesi, R., Veith, G. M., Johansson, P., Edström, K. & Vegge, T. Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ. Sci. 8, 1905–1922 (2015).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Horwitz, G., Rodríguez, C., Factorovich, M. & Corti, H. R. Maximum electrical conductivity of associated lithium salts in solvents for lithium–air batteries. J. Phys. Chem. C 123, 12081–12087 (2019).
Hodgetts, R. Y. et al. Refining universal procedures for ammonium quantification via rapid 1H NMR analysis for dinitrogen reduction studies. ACS Energy Lett. 5, 736–741 (2020).
Xu, C. et al. Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study. J. Mater. Chem. A 2, 7256–7264 (2014).
Takeda, Y., Yamamoto, O. & Imanishi, N. Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes. Electrochemistry 84, 210–218 (2016).
Rakov, D. A. et al. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat. Mater. 19, 1096–1101 (2020).
Bard, A. J. & Faulkner, L. R. in Electrochemical Methods: Fundamentals and Applications 2nd edn, Ch. 13 (John Wiley & Sons, 2001).
Nilsson, V., Bernin, D., Brandell, D., Edström, K. & Johansson, P. Interactions and transport in highly concentrated LiTFSI-based electrolytes. ChemPhysChem 21, 1166–1176 (2020).
Lu, Y. et al. Stable cycling of lithium metal batteries using high transference number electrolytes. Adv. Energy Mater. 5, 1402073 (2015).
Haj Ibrahim, S. et al. Insight into cathode microstructure effect on the performance of molten carbonate fuel cell. J. Power Sources 491, 229562 (2021).
Hodgetts, R. Y., Du, H.-L., Nguyen, T. D., MacFarlane, D. & Simonov, A. N. Electrocatalytic oxidation of hydrogen as an anode reaction for the Li-mediated N2 reduction to ammonia. ACS Catal. 12, 5231–5246 (2022).
Acknowledgements
We acknowledge funding of this work by the Australian Research Council (Discovery Project DP200101878, Centre of Excellence for Electromaterials Science CE140100012, Future Fellowship to A.N.S. (FT200100317)) and the Australian Renewable Energy Agency (‘Renewable Hydrogen for Export’ project 2018RND/009 DM015); and the Monash Centre for Electron Microscopy, Monash X-ray platform and Monash Analytical platform for providing access to the physical characterization and spectroscopic facilities. We thank Nippon Shokubai for a gift of LiFSI and F. Shanks for his assistance with the Fourier-transform infrared attenuated total reflectance spectroscopic measurements.
Author information
Authors and Affiliations
Contributions
H.-L.D. conceived and did the electrochemical experiments, viscosity and conductivity measurements and co-wrote the manuscript. M.C. did the XRD and Fourier-transform infrared attenuated total reflectance spectroscopic analyses, and assisted with monitoring long-term experiments. R.Y.H. contributed to the reproducibility studies and performed the 1H NMR analysis of ammonia. P.V.C. collected and analysed XPS data. C.K.N. did nitrite/nitrate measurements, and the SEM and EDS analyses. K.M. contributed to conductivity measurements and collected the NMR data for the electrolyte stability. D.R.M. and A.N.S. conceived the experiments, directed the project and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.-L.D., D.R.M. and A.N.S. are inventors on an Australian provisional patent application that covers aspects of the work reported here, and which has been licensed to Jupiter Ionics. D.R.M. and A.N.S. have minority equity ownership, as well as management and consulting roles, in Jupiter Ionics.
Peer review
Peer review information
Nature thanks anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–40, Supplementary Tables 1–18 and references.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, HL., Chatti, M., Hodgetts, R.Y. et al. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 609, 722–727 (2022). https://doi.org/10.1038/s41586-022-05108-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05108-y
This article is cited by
-
Cascade electrosynthesis of LiTFSI and N-containing analogues via a looped Li–N2 battery
Nature Catalysis (2024)
-
Phenol as proton shuttle and buffer for lithium-mediated ammonia electrosynthesis
Nature Communications (2024)
-
Lithium-mediated nitrogen reduction to ammonia via the catalytic solid–electrolyte interphase
Nature Catalysis (2024)
-
Long-term continuous ammonia electrosynthesis
Nature (2024)
-
Calcium-mediated nitrogen reduction for electrochemical ammonia synthesis
Nature Materials (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.