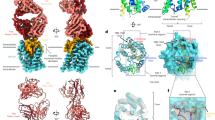
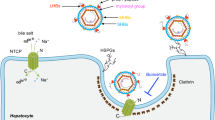
Abstract
Chronic infection with hepatitis B virus (HBV) affects more than 290 million people worldwide, is a major cause of cirrhosis and hepatocellular carcinoma, and results in an estimated 820,000 deaths annually1,2. For HBV infection to be established, a molecular interaction is required between the large glycoproteins of the virus envelope (known as LHBs) and the host entry receptor sodium taurocholate co-transporting polypeptide (NTCP), a sodium-dependent bile acid transporter from the blood to hepatocytes3. However, the molecular basis for the virus–transporter interaction is poorly understood. Here we report the cryo-electron microscopy structures of human, bovine and rat NTCPs in the apo state, which reveal the presence of a tunnel across the membrane and a possible transport route for the substrate. Moreover, the cryo-electron microscopy structure of human NTCP in the presence of the myristoylated preS1 domain of LHBs, together with mutation and transport assays, suggest a binding mode in which preS1 and the substrate compete for the extracellular opening of the tunnel in NTCP. Our preS1 domain interaction analysis enables a mechanistic interpretation of naturally occurring HBV-insusceptible mutations in human NTCP. Together, our findings provide a structural framework for HBV recognition and a mechanistic understanding of sodium-dependent bile acid translocation by mammalian NTCPs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps and structure coordinates have been deposited in the Electron Microscopy Data Bank and the PDB, respectively, under the accession codes EMD-31837 and 7VAD (human NTCP(Q261A)–Fab); EMD-31838 and 7VAE (bovine NTCP(Q261A)–Fab); EMD-31839 and 7VAF (rat NTCP(Q261A)–Fab); EMD-31840 and 7VAG (human NTCP–Fab–myr-preS1); and EMD-32759 and 7WSI (human NTCP(WT)–Fab).
References
The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403 (2018).
Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections (World Health Organization, 2021).
Yan, H. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1, e00049 (2012).
Winer, B. Y. & Ploss, A. Determinants of hepatitis B and delta virus host tropism. Curr. Opin. Virol. 13, 109–116 (2015).
Watashi, K. & Wakita, T. Hepatitis B virus and Hepatitis D virus entry, species specificity, and tissue tropism. Cold Spring Harb. Perspect. Med. 5, a021378 (2015).
Urban, S., Bartenschlager, R., Kubitz, R. & Zoulim, F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology 147, 48–64 (2014).
Leistner, C. M., Gruen-Bernhard, S. & Glebe, D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell. Microbiol. 10, 122–133 (2008).
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46, 1759–1768 (2007).
Ni, Y. et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146, 1070–1083 (2014).
Appelman, M. D., Wettengel, J. M., Protzer, U., Oude Elferink, R. P. J. & van de Graaf, S. F. J. Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1866, 158960 (2021).
Li, M. et al. Apical sodium-dependent bile acid transporter, drug target for bile acid related diseases and delivery target for prodrugs: current and future challenges. Pharmacol. Ther. 212, 107539 (2020).
Anwer, M. S. & Stieger, B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch. 466, 77–89 (2014).
Li, W. The hepatitis B virus receptor. Annu. Rev. Cell Dev. Biol. 31, 125–147 (2015).
Bogomolov, P. et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J. Hepatol. 65, 490–498 (2016).
Passioura, T. et al. De novo macrocyclic peptide inhibitors of hepatitis B virus cellular entry. Cell Chem. Biol. 25, 906–915 (2018).
Yan, H. et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 87, 7977–7991 (2013).
Takeuchi, J. S. et al. A Single adaptive mutation in sodium taurocholate cotransporting polypeptide induced by hepadnaviruses determines virus species specificity. J. Virol. 93, e01432-18 (2019).
Jacquet, S. et al. Evolution of hepatitis B virus receptor NTCP reveals differential pathogenicities and species specificities of hepadnaviruses in primates, rodents, and bats. J. Virol. 93, e01738-18 (2019).
Muller, S. F., Konig, A., Doring, B., Glebe, D. & Geyer, J. Characterisation of the hepatitis B virus cross-species transmission pattern via Na+/taurocholate co-transporting polypeptides from 11 New World and Old World primate species. PLoS ONE 13, e0199200 (2018).
Wang, P. et al. Genetic variations of NTCP are associated with susceptibility to HBV infection and related hepatocellular carcinoma. Oncotarget 8, 105407–105424 (2017).
Fu, L., Hu, H., Liu, Y., Jing, Z. & Li, W. Woodchuck sodium taurocholate cotransporting polypeptide supports low-level hepatitis B and D virus entry. Virology 505, 1–11 (2017).
Saito, S. et al. Catalog of 238 variations among six human genes encoding solute carriers (hSLCs) in the Japanese population. J. Hum. Genet. 47, 576–584 (2002).
Zhou, X. et al. Structural basis of the alternating-access mechanism in a bile acid transporter. Nature 505, 569–573 (2014).
Hu, N. J., Iwata, S., Cameron, A. D. & Drew, D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 478, 408–411 (2011).
Lee, C. et al. A two-domain elevator mechanism for sodium/proton antiport. Nature 501, 573–577 (2013).
Fang, S. et al. Molecular mechanism underlying transport and allosteric inhibition of bicarbonate transporter SbtA. Proc. Natl Acad. Sci. USA 118, e2101632118 (2021).
Bruss, V., Hagelsten, J., Gerhardt, E. & Galle, P. R. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 218, 396–399 (1996).
Gripon, P., Leseyec, J., Rumin, S. & Guguenguillouzo, C. Myristylation of the hepatitis-B virus large surface protein is essential for viral infectivity. Virology 213, 292–299 (1995).
Schulze, A., Schieck, A., Ni, Y., Mier, W. & Urban, S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 84, 1989–2000 (2010).
Kirstgen, M. et al. Selective hepatitis B and D virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci. Rep. 10, 21772 (2020).
Grosser, G., Muller, S. F., Kirstgen, M., Doring, B. & Geyer, J. Substrate specificities and Inhibition pattern of the solute carrier family 10 members NTCP, ASBT and SOAT. Front. Mol. Biosci. 8, 689757 (2021).
Yan, H. et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J. Virol. 88, 3273–3284 (2014).
Konig, A. et al. Kinetics of the bile acid transporter and hepatitis B virus receptor Na+/taurocholate cotransporting polypeptide (NTCP) in hepatocytes. J. Hepatol. 61, 867–875 (2014).
Hu, H. H. et al. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut 65, 1514–1521 (2016).
Peng, L. et al. The p.Ser267Phe variant in SLC10A1 is associated with resistance to chronic hepatitis B. Hepatology 61, 1251–1260 (2015).
Zou, T. T., Zhu, Y., Wan, C. M. & Liao, Q. Clinical features of sodium-taurocholate cotransporting polypeptide deficiency in pediatric patients: case series and literature review. Transl. Pediatr. 10, 1045–1054 (2021).
Dong, C. et al. Clinical and histopathologic features of sodium taurocholate cotransporting polypeptide deficiency in pediatric patients. Medicine 98, e17305 (2019).
Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 543–572 (2016).
Lu, P. H. et al. Dissecting the conformational dynamics of the bile acid transporter homologue ASBTNM. J. Mol. Biol. 433, 166764 (2021).
Noppes, S. et al. Homo- and heterodimerization is a common feature of the solute carrier family SLC10 members. Biol. Chem. 400, 1371–1384 (2019).
Bijsmans, I. T., Bouwmeester, R. A., Geyer, J., Faber, K. N. & van de Graaf, S. F. Homo- and hetero-dimeric architecture of the human liver Na+-dependent taurocholate co-transporting protein. Biochem. J. 441, 1007–1015 (2012).
Fukano, K. et al. Troglitazone impedes the oligomerization of sodium taurocholate cotransporting polypeptide and entry of hepatitis B virus Into hepatocytes. Front. Microbiol. 9, 3257 (2018).
Iwamoto, M. et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl Acad. Sci. USA 116, 8487–8492 (2019).
Jaenecke, F. et al. Generation of conformation-specific antibody fragments for crystallization of the multidrug resistance transporter MdfA. Methods Mol. Biol. 1700, 97–109 (2018).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We thank A. Tsutsumi, H. Yanagisawa, Y. Kise, Y. Sakamaki, M. Kikkawa, Y. Sugita and D. Im for their help in cryo-EM data collection, T. Tomita for allowing us to use the ultracentrifuge, and S.-Y. Park for discussions. Preliminary cryo-EM screening for antibody as a fiducial marker was performed using a Glacios at the RIKEN SPring-8 Center. This work was supported by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant numbers 19H03164 (U.O.), 19H00976 (T.S.), 18K05334 (N.N.) and 19H00923 (S.I. and N.N.)). This work is partially supported by the Knut and Alice Wallenberg foundation (D.D.), the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from Japan Agency for Medical Research and Development (AMED) under grant numbers JP19am0101115 (support no. 1570, 1846, 1848) and JP21am0101079 (S.I. and N.N.), an AMED Research Program on Emerging and Re-emerging Infectious Disease (20fk0108270h0001) (T.N.), the JST Core Research for Evolutional Science and Technology (JPMJCR20HA) (T.N.), JSPS Core-to-Core Program A (T.N.), the Joint Research Project of the Institute of Medical Science, the University of Tokyo (T.N.) and the Joint Usage/Research Center Program of Institute for Frontier Life and Medical Sciences, Kyoto University (N.N. and T.N.)
Author information
Authors and Affiliations
Contributions
J.A., N.N. and U.O. designed the experiments. J.A., H.I., Y.N., Y.S., M.O., D.D., N.N. and U.O. prepared recombinant proteins. K.L., T.U., S.I. and N.N. generated antibodies. J.A., K.T.K., Y.F.-F., Z.Z., M.Y., T.N., H.S., N.N. and U.O. performed cryo-EM analyses. J.A. and H.I performed the in vitro preS1 binding assay. K.T.K. performed the transport assay. J.A., D.D., N.N. and U.O. wrote the paper with assistance from all of the authors. S.I., T.S., N.N. and U.O. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Christoph Seeger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sequence alignment of NTCP and ASBT.
Sequence alignment of human, bovine, and rat NTCPs and human ASBT, as well as bacterial homologues from Yersinia frederiksenii (ASBTYf) and Neisseria meningitidis (ASBTNM), were calculated using Clustal Omega. Coloured bars indicate locations of the transmembrane helices in the human NTCP. Residues involved in myr-preS1 binding are indicated by red boxes. Residues involved in Na binding (Na1 and Na2 sites) are highlighted in cyan and orange, respectively. Residues near the unmodelled lipid densities in the human NTCP–Fab structure and near the TCA molecule in the ASBTNM structure are highlighted in pink and yellow, respectively. Residues forming TM1 in bASBTs are boxed with black lines.
Extended Data Fig. 2 Sample preparation of mammalian NTCP.
a–c, Representative SEC of human (a), bovine (b), and rat (c) NTCP(Q261A). Absorbance profiles (top) and SDS–PAGE analysis of the peak fractions stained with CBB (bottom) are shown. Pooled fractions are shown as bars. Absorbances at 280 nm and 260 nm are shown as solid and dashed lines, respectively. Purifications were performed four, one, and four times for human, bovine, and rat NTCP, respectively. Uncropped blots are shown in Supplementary Fig. 1.
Extended Data Fig. 3 Cryo-EM analysis of mammalian NTCP–Fab complexes.
a–d, Data processing workflow of cryo-EM analysis of human (a), bovine (b), and rat (c) NTCP–YN69202Fab complexes and human NTCP–YN69202Fab complex with myr-preS1 (d). Representative motion-corrected micrograph (out of 5,434 (a), 2,712 (b), 9,932 (c), and 7,496 (d) total micrographs), 2D class averages, 3D class averages, gold-standard FSC curves of the final 3D reconstruction (resolution cut-off at FSC = 0.143), and the final 3D map (coloured according to the local resolution) are shown. 2D class averages were calculated using refined particles that were used for the final reconstruction. 3D classes selected for the following analyses are indicated with red dotted boxes.
Extended Data Fig. 4 Cryo-EM density maps of mammalian NTCPs in complex with Fab.
a, Overall structures of the human (left), bovine (middle), and rat (right) NTCP–YN69202Fab complexes. Cryo-EM maps (top) and ribbon models (bottom) are shown. NTCP and heavy and light chains of Fab are shown in green, cyan, and blue, respectively. b, Cryo-EM density maps of the rat NTCP–YN69202Fab complex around each TM helix of NTCP are shown.
Extended Data Fig. 5 Structural comparison between mammalian NTCP and bASBTs.
a, Structural comparison of human NTCP(Q261A), ASBTYf(E254A) (PDB ID: 4N7X, outward-open), wild-type ASBTYf (PDB ID: 4N7W, inward-open), and wild-type ASBTNM (PDB ID: 3ZUX, inward-open). Each structure was viewed from the extracellular side (top) and parallel to the membrane (bottom). RMSD values of each ASBT structure against the human NTCP structure are indicated (top). b, Structural comparison of the core (left) and panel (right) domains of NTCP and ASBT. Structures of human NTCP(Q261A) (light green), ASBTYf(E254A) (orange), wild-type ASBTYf (cyan), and wild-type ASBTNM (blue) are superposed for each domain. RMSD values of the ASBT structures against the human NTCP structure are indicated (top). c, Close-up view of sodium-binding sites. Na1 (top) and Na2 (bottom) binding sites in the structure of ASBTNM (left) and the corresponding sites of human NTCP (right) are shown. Side chains of residues involved in Na+ binding are labelled and shown as stick representations.
Extended Data Fig. 6 Sodium-dependent transport of NTCP constructs used in this study.
Michaelis–Menten transport kinetics of human NTCP wild type (circle)- and Q261A mutant (triangle)-mediated [3H]-TCA uptake. The specific uptake was calculated by subtracting the sodium-independent and endogenous TCA transport activities from the total uptake, as detailed in Methods. Data represent mean ± standard deviation of three independent measurements. The observed Michaelis constant, Km, for [3H]-TCA is ~9.5 µM (wild type). Estimation of accurate Km value of the Q261A mutant for [3H]-TCA is technically impossible owing to the low transport activity. The Vmax value of the Q261A-mediated uptake was 349.2 ± 103.3 pmol min−1 mg−1, which is fivefold lower than the corresponding value for wild type (1791 ± 154.7 pmol min−1 mg−1).
Extended Data Fig. 7 Structural comparison between wild-type and Q261A-mutant human NTCP.
a, Representative SEC of human NTCP (WT). Absorbance profiles (top) and SDS–PAGE analysis of the peak fractions stained with CBB (bottom) are shown. Pooled fractions are shown as bars. Absorbances at 280 nm and 260 nm are shown as solid and dashed lines, respectively. Purifications were performed nine times with similar results. The uncropped blot is shown in Supplementary Fig. 1. b, Data processing workflow of cryo-EM analysis of human NTCP (wild type)-YN69202Fab complex. Representative motion-corrected micrograph, 2D class averages, 3D class averages, gold-standard FSC curves of the final 3D reconstruction (resolution cut-off at FSC = 0.143), and the final 3D map (coloured according to the local resolution) are shown. 2D class averages were calculated using refined particles that were used for the final reconstruction. 3D classes selected for the following analyses are indicated with red dotted boxes. c, Structural comparison of wild-type and Q261A-mutant human NTCP. Structures of human NTCP (wild type) (grey) and human NTCP(Q261A) (light green) are superposed (upper left). Na1 (upper right) and Na2 (bottom right) binding sites in the structure of human NTCP (wild type) and human NTCP(Q261A) are shown. Side chains of residues involved in Na+ binding are labelled and shown as stick representations. Close-up view of human NTCP (wild type) marked with a black rectangle is shown (bottom left). The two observed densities are indicated in orange. Side chains of residues near the densities are shown as stick representations. Crossover regions of TM3 and TM8 is indicated by a black dotted circle.
Extended Data Fig. 8 NTCP–preS1 binding interface.
a, Topology diagrams of HBV envelope glycoproteins LHBs. b, Sequences of preS1 mutants used in Fig. 3c. Sequence of wild-type preS1 (2–48) and the residue numbers are indicated (top). Dots in the sequence indicate the same residues as the wild type. c, Structural comparison of predicted preS1-binding sites, patch 1 (left), and patch 2 (right), among mammalian NTCPs. Superposition of human (light green), bovine (cyan), and rat (magenta) NTCPs with patch 1 (in TM2–3 loop) and patch 2 (in TM5), indicated with rectangles (top). A detailed view and sequence of each site are shown (bottom).
Extended Data Fig. 9 Cryo-EM densities in the tunnel in NTCP.
Cryo-EM density maps for the human NTCP–YN69202Fab complex in the presence (green) and absence (orange) of myr-preS1. Only the densities in the channel are displayed.
Supplementary information
Rights and permissions
About this article
Cite this article
Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04845-4
This article is cited by
-
The crossing of two unwound transmembrane regions that is the hallmark of the NhaA structural fold is critical for antiporter activity
Scientific Reports (2024)
-
Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP
Nature Communications (2024)
-
Structural basis of hepatitis B virus receptor binding
Nature Structural & Molecular Biology (2024)
-
A large expert-curated cryo-EM image dataset for machine learning protein particle picking
Scientific Data (2023)
-
Targeted viral adaptation generates a simian-tropic hepatitis B virus that infects marmoset cells
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.