Most cases of SARS-CoV-2 infection are mild, but a proportion of people progress to severe COVID-19 for reasons that are not entirely clear. Some factors associated with the severe illness are known. For example, people with severe COVID-19 often have other pre-existing conditions, such as coronary artery disease, hypertension and diabetes1. Unsurprisingly, compromised antiviral defences can also lead to life-threatening illness, as is the case for around 15% of people with severe COVID-19 who have a deficiency in what is called the type I interferon pathway2. Indeed, it has been known since the discovery of this pathway in 1957 that it directly interferes with viral replication in an infected cell3.
Read the paper: Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells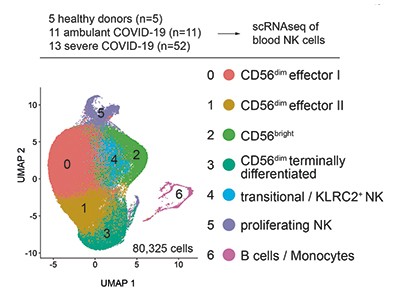
There might also be other mechanisms at play in antiviral defences, such as a role for the natural killer (NK) cells of the immune system. Writing in Nature, Witkowski et al.4 report that people with severe COVID-19 have higher than normal levels of an anti-inflammatory molecule, transforming growth factor-β1 (TGF‑β1), in their bloodstream, and that this is associated with impaired antiviral defence by NK cells and defective immune-system control of SARS‑CoV-2.
Natural killer cells are white blood cells that are part of the innate branch of the immune system. They make key contributions to tackling viral infections, and their rapid activation is linked to the efficient control of several types of virus5. NK cells can eliminate virus-infected cells in various ways — by releasing cytotoxic granules (termed degranulation) that directly kill the virus-infected cells, or by shaping both the innate and adaptive branches of immune defence by producing molecules called cytokines and chemokines, which can modulate the behaviour of other immune cells. Previous studies6–10 monitored the responses of NK cells in individuals with COVID-19, and reported lower than normal levels of NK cells in people with the disease, but the precise role of these cells in severe COVID-19 was unclear.
Witkowski et al. analysed clinical samples, and found a correlation between a rapid decline in the level of virus (the viral load) and high levels of NK cells in the bloodstream at the beginning of the infection. This correlation was not observed for the T cells or B cells of the immune system. The drastic decrease in NK cells in the bloodstream over the course of infection might be due to their death by a process called apoptosis7, or their recruitment from the bloodstream to the lungs.
Crucially, Witkowski et al. report that NK cells from healthy individuals can directly kill SARS-CoV-2-infected cells in vitro, but that NK cells from people with moderate or severe COVID-19 had impaired cell-killing activity (Fig. 1). These defective NK cells express higher-than-normal levels of the cytotoxic molecules that kill cells, but are impaired in their ability to bind to target cells and to export their cytotoxic granules at the cell surface. In addition, NK cells from people with severe COVID-19 were less able to produce cytokines and chemokines than were such cells from healthy donors or from infected people who have no or minimal COVID-19 symptoms.
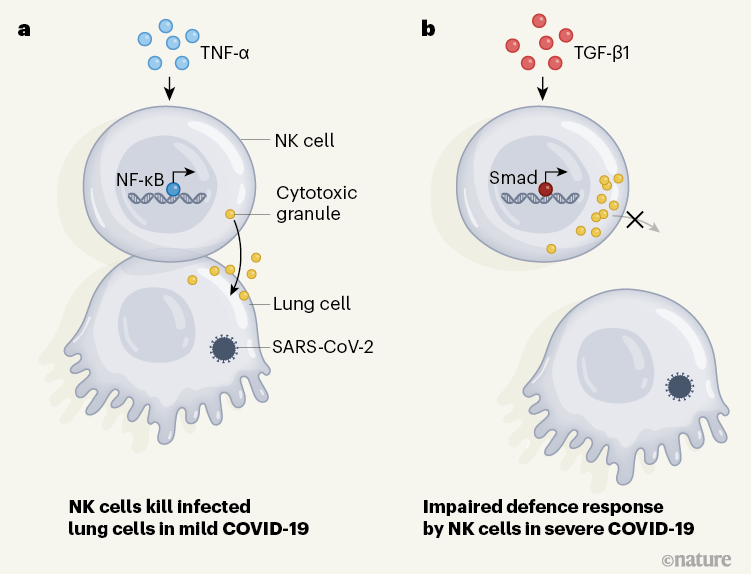
Figure 1 | Characterizing immune responses associated with COVID-19. Immune cells called natural killer (NK) cells can help to tackle viral infections by releasing cytotoxic granules that can kill infected cells. a, Previous work7 indicates that, in cases of mild COVID-19, NK cells express genes that are typically induced through the protein TNF-α pathway. This type of gene-expression profile arises through the action of transcription factors such as the protein NF-κB, and it aids the recovery of function of NK cells7. b, Witkowski et al.4 analysed samples of NK cells from individuals with severe COVID-19, and report that these cells had a gene-expression pattern characteristic of that driven by the protein TGF-β1. Gene expression mediated by this pathway depends on transcription factors that include Smad proteins. The authors report that NK cells from people with severe COVID-19 are impaired in their ability to bind to infected cells and to release cytotoxic granules. This finding provides insights into the factors driving severe COVID-19.
To dissect the mechanisms underlying the dysfunction of NK cells, Witkowski et al. assessed gene expression by carrying out single-cell RNA sequencing of these cells from people with COVID-19 of differing degrees of severity. The authors found, consistently with another study7 reporting a similar transcriptional analysis of NK cells from people with COVID-19, that the cells had a gene-expression pattern characteristic of that driven by type I interferons. This gene-expression signature was more prominent in NK cells from people with severe rather than milder forms of COVID-19. Moreover, a pattern of gene expression characteristic of that driven by the protein TNF-α in NK cells has been described7 for mild cases of COVID-19. Witkowski et al. also reveal an increase in the expression of genes controlled by TGF‑β1 in NK cells purified from either the blood or the lungs of people infected with SARS-CoV-2. This increase seems to correlate with the patients’ symptoms, with a higher prevalence of TGF‑β1-driven gene expression corresponding to more severe illness.
TGF-β1 is a cytokine with a central role in tissue remodelling, but it also suppresses the functioning of NK cells11. TGF-β1 limits the ability of NK cells to control SARS-CoV-2 replication as tested in vitro. When NK cells from healthy individuals were treated with TGF-β1 in vitro, the cells were less able to form connections with infected target cells and were less able to degranulate and produce cytokines.
Signs of self-sustained inflammatory circuits in severe COVID pneumonia
Witkowski and colleagues report high levels of TGF-β1 in the blood of people with severe COVID-19, but not in those with no or minimal signs of the disease. Notably, the authors reveal that serum from people with severe COVID-19 can limit, to some extent, the in vitro degranulation and control of viral replication mediated by NK cells from healthy donors, and that this effect can be prevented by adding an antibody that blocks TGF-β1. Together, these findings indicate a correlation between severe COVID-19, the presence of a high level of TGF-β1 in the bloodstream and an impaired defensive response by NK cells.
Several questions remain. The first is whether NK cells are necessary to fight SARS‑CoV-2 infection. Several studies of mouse models of respiratory viral infections, such as influenza A virus, respiratory syncytial virus and Sendai virus, have shown that NK cells contribute to virus control and aid host survival5. Consistent with this, the only characteristic shared by people with various types of NK-cell deficiency is a predisposition to viral infections, especially of herpesvirus12. The fact that type I interferons are strong activators of NK cells also suggests a link between deficiencies in type I interferons and impaired NK-cell-mediated antiviral immunity. All these findings, together with the inverse correlation between the number of functional NK cells and the severity of COVID-19, support a role for these cells in controlling SARS-CoV-2 infection, but formal proof of this hypothesis remains to be obtained.
Natural killer cells lull tumours into dormancy
A second question to be addressed is, what are the mechanisms underlying the high production of TGF-β1 during COVID-19? SARS‑CoV-2 contains a spike protein that interacts with the ACE2 receptor on human cells1. This receptor is an enzyme that converts the protein angiotensin II (AngII) to a peptide called Ang1-7. The enzymatic activity of ACE2 is disturbed during SARS-CoV-2 infection, resulting in a rise in AngII levels that promotes TGF-β1 expression1. Thus, excessive damage to lung tissue caused by a high viral load might induce a high level of TGF-β1, which could affect NK cells. Moreover, TGF-β1 is known to promote a type of tissue damage called fibrosis, which is a hallmark of severe COVID-19. A rise in the level of AngII is a common feature of coronary artery disease, hypertension and diabetes13, and high levels of TGF-β1 are observed in obesity14. It will therefore be interesting to investigate whether the association of such conditions with severe COVID-19 involves defective functioning of NK cells mediated by TGF-β1.
Ways of manipulating NK cells are of increasing interest in anticancer therapy, using approaches such as immune-checkpoint inhibitors, NK-cell ‘engagers’ and off-the-shelf infusions of NK cells15. The importance of NK cells in the control of viruses should similarly prompt the design of innovative treatments based on harnessing these cells to fight viral infections. In the case of severe COVID-19, the high levels of TGF-β1 present might compromise strategies to boost the activity of NK cells. Instead, the use of TGF-β1 blockers16 might be a way to promote NK-cell-mediated antiviral defence and prevent lung fibrosis, although the safety of such treatment in this context is a concern that should be addressed. In addition, monitoring TGF-β1 levels in plasma, along with TNF-α-mediated gene expression in NK cells from the bloodstream7, might help to predict the outcome of infection and to guide a patient’s care appropriately.

 Read the paper: Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells
Read the paper: Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells
 Signs of self-sustained inflammatory circuits in severe COVID pneumonia
Signs of self-sustained inflammatory circuits in severe COVID pneumonia
 Natural killer cells lull tumours into dormancy
Natural killer cells lull tumours into dormancy







