Abstract
Human exposure to toxic mercury (Hg) is dominated by the consumption of seafood1,2. Earth system models suggest that Hg in marine ecosystems is supplied by atmospheric wet and dry Hg(ii) deposition, with a three times smaller contribution from gaseous Hg(0) uptake3,4. Observations of marine Hg(ii) deposition and Hg(0) gas exchange are sparse, however5, leaving the suggested importance of Hg(ii) deposition6 ill-constrained. Here we present the first Hg stable isotope measurements of total Hg (tHg) in surface and deep Atlantic and Mediterranean seawater and use them to quantify atmospheric Hg deposition pathways. We observe overall similar tHg isotope compositions, with median Δ200Hg signatures of 0.02‰, lying in between atmospheric Hg(0) and Hg(ii) deposition end-members. We use a Δ200Hg isotope mass balance to estimate that seawater tHg can be explained by the mixing of 42% (median; interquartile range, 24–50%) atmospheric Hg(ii) gross deposition and 58% (50–76%) Hg(0) gross uptake. We measure and compile additional, global marine Hg isotope data including particulate Hg, sediments and biota and observe a latitudinal Δ200Hg gradient that indicates larger ocean Hg(0) uptake at high latitudes. Our findings suggest that global atmospheric Hg(0) uptake by the oceans is equal to Hg(ii) deposition, which has implications for our understanding of atmospheric Hg dispersal and marine ecosystem recovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Hg stable isotope and Hg speciation data that support the findings of this study are available from https://doi.org/10.5281/zenodo.4740464. Source data are provided with this paper.
References
Sunderland, E. M. Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ. Health Perspect. 115, 235–242 (2007).
Lavoie, R. A., Bouffard, A., Maranger, R. & Amyot, M. Mercury transport and human exposure from global marine fisheries. Sci. Rep. 8, 6705 (2018).
Horowitz, H. M. et al. A new mechanism for atmospheric mercury redox chemistry: implications for the global mercury budget. Atmos. Chem. Phys. 17, 6353–6371 (2017).
Travnikov, O. et al. Multi-model study of mercury dispersion in the atmosphere: atmospheric processes and model evaluation. Atmos. Chem. Phys. 17, 5271–5295 (2017).
Zhang, L., Zhou, P., Cao, S. & Zhao, Y. Atmospheric mercury deposition over the land surfaces and the associated uncertainties in observations and simulations: a critical review. Atmos. Chem. Phys. 19, 15587–15608 (2019).
Zhang, Y. et al. A coupled global atmosphere-ocean model for air-sea exchange of mercury: insights into wet deposition and atmospheric redox chemistry. Environ. Sci. Technol. 53, 5052–5061 (2019).
Sheehan, M. C. et al. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull. World Health Organ. 92, 254–269 (2014).
Mason, R. P. & Fitzgerald, W. F. Alkylmercury species in the Equatorial Pacific. Nature 347, 457–459 (1990).
Selin, N. E. Global biogeochemical cycling of mercury: a review. Ann. Rev. Environ. Resour. 34, 43–63 (2009).
Fitzgerald, W. F., Lamborg, C. H. & Hammerschmidt, C. R. Marine biogeochemical cycling of mercury. Chem. Rev. 107, 641–662 (2007).
Li, C. et al. Unequal anthropogenic enrichment of mercury in Earth’s northern and southern hemispheres. ACS Earth Space Chem. 4, 2073–2081 (2020).
Outridge, P. M., Mason, R. P., Wang, F., Guerrero, S. & Heimbürger-Boavida, L. E. Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol. 52, 11466–11477 (2018).
Lamborg, C. H. et al. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512, (2014).
Schartup, A. T. et al. Climate change and overfishing increase neurotoxicant in marine predators. Nature 572, 648–650 (2019).
Sunderland, E. M. & Mason, R. P. Human impacts on open ocean mercury concentrations. Global Biogeochem. Cycles 21, GB4022 (2007).
Amos, H. M. et al. Global biogeochemical implications of mercury discharges from rivers and sediment burial. Environ. Sci. Technol. 48, 9514–9522 (2014).
Zhang, Y. et al. Biogeochemical drivers of the fate of riverine mercury discharged to the global and Arctic oceans. Global Biogeochem. Cycles 29, 854–864 (2015).
Zhang, Y., Jaegle, L., Thompson, L. & Streets, D. G. Six centuries of changing oceanic mercury. Global Biogeochem. Cycles 28, 1251–1261 (2014).
Soerensen, A. L. et al. Elemental mercury concentrations and fluxes in the tropical atmosphere and ocean. Environ. Sci. Technol. 48, 11312–11319 (2014).
Kuss, J., Zülicke, C., Pohl, C. & Schneider, B. Atlantic mercury emission determined from continuous analysis of the elemental mercury sea-air concentration difference within transects between 50°N and 50°S. Global Biogeochem. Cycles 25, GB3021 (2011).
Wang, J., Xie, Z., Wang, F. & Kang, H. Gaseous elemental mercury in the marine boundary layer and air-sea flux in the Southern Ocean in austral summer. Sci. Total Environ. 603–604, 510–518 (2017).
Krabbenhoft, D. P. & Sunderland, E. M. Global change and mercury. Science 341, 1457–1458 (2013).
Enrico, M. et al. Atmospheric mercury transfer to peat bogs dominated by gaseous elemental mercury dry deposition. Environ. Sci. Technol. 50, 2405–2412 (2016).
Demers, J. D., Blum, J. D. & Zak, D. R. Mercury isotopes in a forested ecosystem: implications for air-surface exchange dynamics and the global mercury cycle. Global Biogeochem. Cycles 27, 222–238 (2013).
Obrist, D. et al. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 547, 201–204 (2017).
Chen, J., Hintelmann, H., Feng, X. & Dimock, B. Unusual fractionation of both odd and even mercury isotopes in precipitation from Peterborough, ON, Canada. Geochim. Cosmochim. Acta 90, 33–46 (2012).
Fu, X. W. et al. Mass-independent fractionation of even and odd mercury isotopes during atmospheric mercury redox reactions. Environ. Sci. Technol. 55, 10164–10174 (2021).
Enrico, M. et al. Holocene atmospheric mercury levels reconstructed from peat bog mercury stable isotopes. Environ. Sci. Technol. 51, 5899–5906 (2017).
Štrok, M., Baya, P. A. & Hintelmann, H. The mercury isotope composition of Arctic coastal seawater. C.R. Geosci. 347, 368–376 (2015).
Motta, L. C. et al. Mercury cycling in the North Pacific subtropical gyre as revealed by mercury stable isotope ratios. Global Biogeochem. Cycles 33, 777–794 (2019).
Bowman, K. L., Lamborg, C. H. & Agather, A. M. A global perspective on mercury cycling in the ocean. Sci. Total Environ. 710, 136166 (2020).
Cossa, D., Averty, B. & Pirrone, N. The origin of methylmercury in open Mediterranean waters. Limnol. Oceanogr. 54, 3 (2009).
Heimbürger, L.-E. et al. Methyl mercury distributions in relation to the presence of nanoand picophytoplankton in an oceanic water column (Ligurian Sea, north-western Mediterranean). Geochim. Cosmochim. Acta 74, 5549–5559 (2010).
Cossa, D. et al. Mercury distribution and transport in the North Atlantic Ocean along the GEOTRACES-GA01 transect. Biogeosciences 15, 2309–2323 (2018).
Gehrke, G. E., Blum, J. D. & Meyers, P. A. The geochemical behavior and isotopic composition of Hg in a mid-Pleistocene western Mediterranean sapropel. Geochim. Cosmochim. Acta 73, 1651–1665 (2009).
Ogrinc, N., Hintelmann, H., Kotnik, J., Horvatl, M. & Pirrone, N. Sources of mercury in deep-sea sediments of the Mediterranean Sea as revealed by mercury stable isotopes. Sci. Rep. 9, 11626 (2019).
Zhang, Y. et al. Biogeochemical drivers of the fate of riverine mercury discharged to the global and Arctic oceans. Global Biogeochem. Cycles 29, 854–864 (2015).
Shah, V. & Jaeglé, L. Subtropical subsidence and surface deposition of oxidized mercury produced in the free troposphere. Atmos. Chem. Phys. 17, 8999–9017 (2017).
Rolison, J. M., Landing, W. M., Cohen, M. D., Luke, W. & Salters, V. J. M. Isotopic composition of species-specific atmospheric Hg in a coastal environment. Chem. Geol. 336, 37–49 (2012).
Washburn, S. J., Blum, J. D., Motta, L. C., Bergquist, B. A. & Weiss-Penzias, P. Isotopic composition of Hg in fogwaters of coastal California. Environ. Sci. Technol. Lett. 8, 3–8 (2020).
Yu, B. et al. New evidence for atmospheric mercury transformations in the marine boundary layer from stable mercury isotopes. Atmos. Chem. Phys. 20, 9713–9723 (2020).
Demers, J. D., Sherman, L. S., Blum, J. D., Marsik, F. J. & Dvonch, J. T. Coupling atmospheric mercury isotope ratios and meteorology to identify sources of mercury impacting a coastal urban-industrial region near Pensacola, Florida, USA. Global Biogeochem. Cycles 29, 1689–1705 (2015).
Fu, X. et al. Isotopic composition of gaseous elemental mercury in the marine boundary layer of East China Sea. J. Geophys. Res. Atmos. 123, 7656–7669 (2018).
Semeniuk, K. & Dastoor, A. Development of a global ocean mercury model with a methylation cycle: outstanding issues. Global Biogeochem. Cycles 31, 400–433 (2017).
Soerensen, A. L. et al. An improved global model for air-sea exchange of mercury: high concentrations over the North Atlantic. Environ. Sci. Technol. 44, 8574–8580 (2010).
Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry (EPA, 2002).
Cutter, G. J. et al. Sampling and Sample-handling Protocols for GEOTRACES Cruises. Version 3.0. (2017).
Sun, R., Enrico, M., Heimbürger, L.-E., Scott, C. & Sonke, J. E. A double-stage tube furnace-acid-trapping protocol for the pre-concentration of mercury from solid samples for isotopic analysis. Anal. Bioanal.Chem. 405, 6771–6781 (2013).
Heimbürger, L. E. et al. Shallow methylmercury production in the marginal sea ice zone of the central Arctic Ocean. Sci. Rep. 5, 10318 (2015).
Jiskra, M., Sonke, J. E., Agnan, Y., Helmig, D. & Obrist, D. Insights from mercury stable isotopes on terrestrial-atmosphere exchange of Hg(0) in the Arctic tundra. Biogeosciences 16, 4051–4064 (2019).
Blum, J. D. & Bergquist, B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal.Chem. 388, 353–359 (2007).
Jiskra, M. et al. Mercury deposition and re-emission pathways in boreal forest soils investigated with Hg isotope signatures. Environ. Sci. Technol. 49, 7188–7196 (2015).
Bergquist, B. A. & Blum, J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318, 417–420 (2007).
Vermeesch, P. IsoplotR: a free and open toolbox for geochronology. Geosci. Front. 9, 1479–1493 (2018).
Blum, J. D. & Johnson, M. W. Recent developments in mercury stable isotope analysis. Rev. Mineral. Geochem. 82, 733–757 (2017).
Acknowledgements
This work was supported by research grants ANR-17-CE34-0010 MERTOX to D.P., FP7-IDEAS-ERC grant no. 258537, and H2020 ERA-PLANET grant no. 689443 via the iCUPE and iGOSP project to J.E.S., Chantier Arctique Francais funding via the Pollution in the Arctic System Project to J.E.S. and L.E.H.B., H2020 Marie Skłodowska-Curie grant no. 657195 and Swiss National Science Foundation grant PZ00P2_174101 to M.J., APOG DECOMAR, MISTRALS AT P&C and the AXA RF grants to L.E.H.B., and the French National Research Agency (ANR-13-BS06-0014, ANR-12-PDOC-0025-01), the French National Centre for Scientific Research (CNRS-LEFE-CYBER), the LabexMER (ANR-10-LABX-19), and Ifremer. We are grateful to G. Sarthou and P. Lherminier, chief scientists of the 2014 GEOVIDE cruise, and to H. Planquette for coordinating clean sampling. We thank M. Rutgers van der Loeff, T. Kanzow and the Alfred-Wegener-Institute for Polar and Marine Research for organizing the 2016 GRIFF cruise. We thank E. de Saint-Léger and F. Pérault of the technical division of INSU for support with operations at sea. We thank L. Laffont for laboratory management and O. Grosso and D. Malengros for technical assistance. We thank L. Metral from MARBEC and F. Ménard from MIO for providing tuna fish samples from the Mediterranean Sea. We thank J. Kuss and H. Horowitz for discussion on gas exchange model parameterization. We thank the captains, crew and sampling teams onboard the RV Antedon II, RV Pourquoi Pas? and FS Polarstern for their support at sea. Thanks also go to the shipboard participants, captain and crew of the N/O l’Atalante for obtaining sediment samples from the 2015 VESPA cruise. VESPA was funded by the French Ministry of Research and Higher Education, with support from the governments of New Zealand and New Caledonia.
Author information
Authors and Affiliations
Contributions
L.E.H.B., J.E.S., M.J. and D.P. conceived the study. L.E.H.B., M.J., D.P., M.P., M.V.P., M.M.D. and J.E.S. performed sampling. J.E.S., M.J. and L.E.H.B. developed and applied the tHg isotope pre-concentration methods. J.E.S., M.J., J.M. and J.C. performed isotope measurements. M.M.D., M.V.P., A.D., L.E.H.B., M.J., J.M., D.P. and M.T. performed additional laboratory work. M.J., J.E.S. and L.E.H.B. analysed the data. J.E.S. and M.J. wrote the draft paper, which was improved by contributions from L.E.H.B. and D.P., and commented on by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Summary of marine Hg(ii) deposition and Hg(0) air-sea exchange fluxes.
Gross fluxes (solid arrows, Mg y−1) are based on published model estimates3. Hg(0) exchange is bidirectional, meaning that despite surface ocean Hg(0) supersaturation and large Hg(0) evasion, Hg(0) invasion is substantial. Marine Δ200Hg signatures of 0.04‰ indicate a relatively more important contribution of the atmospheric Hg(0) end-member to marine Hg than current 3D models suggest. This indicates that either 3D model Hg(ii) deposition is overestimated or that Hg(0) invasion is underestimated (black dotted arrows, indicating 2–3 times lower or 2–3 times higher fluxes, required to fit Δ200Hg data).
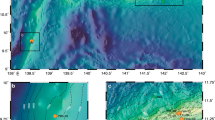
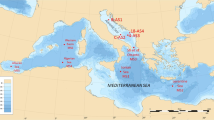
Extended Data Fig. 2 Maps of sampling locations for total and particulate Hg isotopes.
Top: sampling locations K2 in the Mediterranean Sea (purple), Atlantic Ocean (yellow) and Fram Strait (green). Bottom: magnification of the four Mediterranean locations, with main station K2 (large purple circle), and pHg station K1 and Julio (small purple circles), and Endoume pier in Marseille Bay (grey square). Maps were made with Ocean Data View (Schlitzer, Reiner, Ocean Data View, odv.awi.de, 2021).
Extended Data Fig. 3 Latitudinal distribution of Hg(ii) wet deposition.
Annual mean Hg(ii) wet deposition (µg m−2 y−1) at oceanic locations in the northern and southern hemispheres (NH, SH), binned in 5° latitude. Mean values (± standard deviation, SD) were calculated when sufficient data was available per 5° latitude band, and interpolated using polynomial fitting when no data were available (in which case a mean observed SD of 30% was applied). MDN, mercury deposition network; GMOS, global mercury observation system; USA, CAN, PR, United States of America, Canada, Puerto Rico.
Extended Data Fig. 4 Latitudinal distribution of dissolved gaseous Hg (DGM) concentrations.
Mean (± standard deviation) DGM are binned in 5° latitude bands, and equal weight was given to each study. Polar studies, affected by sea ice show unusually high concentrations (mean 219 fM in the Arctic, mean 138 fM around Antarctica) for high latitude waters and were excluded in 5° latitude binning (replaced in calculations by ‘open water only’ DGM data at 55–60°S and 75–80°N).
Extended Data Fig. 5 Atmospheric deposition pathways of the zonal reference model.
a, Marine Hg(ii) gross deposition, Hg(0) gross invasion, Hg(0) gross evasion, and net Hg flux [Hg(ii) deposition + Hg(0) invasion – Hg(0) evasion]; all in µg m−2 y−1 with evasion shown as negative numbers. Hg(0) invasion is driven by observed atmospheric Hg(0) and wind speed. Hg(ii) deposition is dominated by Hg(ii) wet deposition. Hg(0) evasion is driven by DGM concentrations and wind speed. The net Hg evasion trends shows important net deposition in the northern hemisphere, and net evasion in the southern hemisphere. b, Reference model Hg gross deposition fluxes (µg m−2 y−1) as a function of latitude used in estimating marine Δ200Hg in Fig. 4a (main text). Hg(ii) wet deposition observations as in Extended Data Fig. 3; Hg(ii) dry deposition was fixed at 5 µg m−2 y−1, and constrained as 40% of total Hg(ii) deposition38, since no dry deposition observations over oceans exist. Hg(0) invasion (ocean uptake, same as in top panel) is calculated from the observed inter-hemispheric atmospheric gaseous Hg(0) gradient3, wind and sea surface temperature (Copernicus), and the latest Hg(0) air–sea gas exchange model (see Supplementary Information).
Extended Data Fig. 6 Estimated latitudinal variation in Δ200Hg of atmospheric Hg(ii) deposition.
Extended Data Fig. 7 Variation of Δ204Hg in marine samples.
a, Δ204Hg versus Δ200Hg. The dashed line represents the York regression using IsoplotR54 for all marine samples (Δ200Hg = −0.32(±0.06) Δ204Hg + (0.03±0.004), (± se), MSWD = 0.213). b, Δ204Hg boxplot for 5° latitudinal intervals. Marine samples are shown in boxes, where the bold horizontal line represents the median, the boxes the interquartile range, the whiskers 1.5 times the IQR and outliers are represented by dots. The solid line represents the predicted Δ204Hg based on the observational relationship between Δ204Hg and Δ200Hg in terrestrial samples by Blum and Johnson, 201755. The dashed line represents the predicted Δ204Hg derived from the York regression shown in panel a. Δ204Hg data are available for 339 out of 791 marine samples. Note that for pHg and tHg samples presented here, Δ204Hg was not measured because of the low abundance of the 204Hg isotope, and unavailability of a second 1013 Ω amplifier.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–3 and references.
Rights and permissions
About this article
Cite this article
Jiskra, M., Heimbürger-Boavida, LE., Desgranges, MM. et al. Mercury stable isotopes constrain atmospheric sources to the ocean. Nature 597, 678–682 (2021). https://doi.org/10.1038/s41586-021-03859-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03859-8
This article is cited by
-
Recurrent photic zone euxinia limited ocean oxygenation and animal evolution during the Ediacaran
Nature Communications (2023)
-
Inductively coupled plasma mass spectrometry
Nature Reviews Methods Primers (2023)
-
A 1500-year record of mercury isotopes in seal feces documents sea ice changes in the Antarctic
Communications Earth & Environment (2023)
-
Mercury deposition and redox transformation processes in peatland constrained by mercury stable isotopes
Nature Communications (2023)
-
Global change effects on biogeochemical mercury cycling
Ambio (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.