Abstract
The hippocampus has previously been implicated in both cognitive and endocrine functions1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. We simultaneously measured electrophysiological activity from the hippocampus and interstitial glucose concentrations in the body of freely behaving rats to identify an activity pattern that may link these disparate functions of the hippocampus. Here we report that clusters of sharp wave-ripples recorded from the hippocampus reliably predicted a decrease in peripheral glucose concentrations within about 10 min. This correlation was not dependent on circadian, ultradian or meal-triggered fluctuations, could be mimicked with optogenetically induced ripples in the hippocampus (but not in the parietal cortex) and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum, which is the major conduit between the hippocampus and the hypothalamus. Our findings demonstrate that a function of the sharp wave-ripple is to modulate peripheral glucose homeostasis, and offer a mechanism for the link between sleep disruption and blood glucose dysregulation in type 2 diabetes16,17,18.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets used during this study are available from https://buzsakilab.nyumc.org/datasets/TingleyD/.
Code availability
The code used in this study is available from https://github.com/buzsakilab/buzcode.
References
Kanoski, S. E. & Grill, H. J. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 81, 748–756 (2017).
Lavenex, P. & Amaral, D. G. Hippocampal–neocortical interaction: a hierarchy of associativity. Hippocampus 10, 420–430 (2000).
Seto, K., Saito, H., Otsuka, K. & Kawakami, M. Influence of electrical stimulation of the limbic structure on insulin level in rabbit’s plasma. Exp. Clin. Endocrinol. 81, 347–349 (1983).
Endroczi, E., Lissak, K., Bohus, B. & Kovacs, S. The inhibitory influence of archicortical structures on pituitary-adrenal function. Acta Physiol. Acad. Sci. Hung. 16, 17–22 (1959).
Rubin, R. T., Mandell, A. J. & Crandall, P. H. Corticosteroid responses to limbic stimulation in man: localization of stimulus sites. Science 153, 767–768 (1966).
McGaugh, J. L. & Gold, P. E. Hormonal Modulation of Memory. Psychoendocrinology (Academic, 1989).
Gold, P. E. & van Buskirk, R. Effects of posttrial hormone injections on memory processes. Horm. Behav. 7, 509–517 (1976).
McEwen, B. S., Weiss, J. M. & Schwartz, L. S. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 16, 227–241 (1969).
Lathe, R. Hormones and the hippocampus. J. Endocrinol. 169, 205–231 (2001).
Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Bohus, B. in The Hippocampus: Volume 1: Structure and Development (eds Isaacson, R. L. & Pribram, K. H.) 323–353 (Springer, 1975).
Jacobson, L. & Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118–134 (1991).
Hsu, T. M. et al. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 4, e11190 (2015).
Saphier, D. & Feldman, S. Effects of septal and hippocampal stimuli on paraventricular nucleus neurons. Neuroscience 20, 749–755 (1987).
Prutskova, N. P. & Petrov, Yu. A. Electrophysiological investigation of the hippocampal projections to the neurosecretory cells of the supraoptic nucleus of the rat hypothalamus. Neurosci. Behav. Physiol. 20, 194–200 (1990).
Tasali, E., Leproult, R., Ehrmann, D. A. & Van Cauter, E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl Acad. Sci. USA 105, 1044–1049 (2008).
Cappuccio, F. P., D’Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–420 (2010).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Amaral, D. G. & Lavenex, P. in The Hippocampus Book (eds. Anderson, P. et al.) 45–66 (Oxford Univ. Press, 2006).
Cenquizca, L. A. & Swanson, L. W. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J. Comp. Neurol. 497, 101–114 (2006).
Shimazu, T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab. Rev. 3, 185–206 (1987).
Buzsáki, G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015).
Hansen, K. Oscillations in the blood sugar in fasting normal persons. Acta Med. Scand. 57, 27–32 (1923).
Risold, P. Y. & Swanson, L. W. Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev. 24, 115–195 (1997).
Sosa, M., Joo, H. R. & Frank, L. M. Dorsal and ventral hippocampal sharp-wave ripples activate distinct nucleus accumbens networks. Neuron 105, 725–741 (2020).
Tingley, D. & Buzsáki, G. Routing of hippocampal ripples to subcortical structures via the lateral septum. Neuron 105, 138–149 (2020).
Niijima, A. Neural control of blood glucose level. Jpn. J. Physiol. 36, 827–841 (1986).
Britton, S. W. Studies on the conditions of activity in endocrine glands: XVII. The nervous control of insulin secretion. Am. J. Physiol. 74, 291–308 (1925).
Corkill, A. B., Marks, H. P. & White, W. E. Relation of the pituitary gland to the action of insulin and adrenaline. J. Physiol. 612, 193–205 (1933).
Kuo, T., McQueen, A., Chen, T.-C. & Wang, J.-C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 872, 99–126 (2015).
Kim, S. H. & Park, M.-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann. Pediatr. Endocrinol. Metab. 22, 145–152 (2017).
Güemes, A. & Georgiou, P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron. Med. 4, 9 (2018).
Banks, W. A. & Kastin, A. J. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19, 883–889 (1998).
Schulingkamp, R. J., Pagano, T. C., Hung, D. & Raffa, R. B. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 24, 855–872 (2000).
O’Malley, D., Shanley, L. J. & Harvey, J. Insulin inhibits rat hippocampal neurones via activation of ATP-sensitive K+ and large conductance Ca2+-activated K+ channels. Neuropharmacology 44, 855–863 (2003).
Harasta, A. E. et al. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology 40, 1969–1978 (2015).
Somers, V. K., Dyken, M. E., Mark, A. L. & Abboud, F. M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 328, 303–307 (1993).
Alford, F. P. et al. Temporal patterns of integrated plasma hormone levels during sleep and wakefulness. I. Thyroid-stimulating hormone, growth hormone and cortisol. J. Clin. Endocrinol. Metab. 37, 841–847 (1973).
Logothetis, N. K. et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012).
Swanson, R. A., Levenstein, D., McClain, K., Tingley, D. & Buzsáki, G. Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Curr. Opin. Behav. Sci. 32, 126–135 (2020).
Csicsvari, J., Hirase, H., Czurkó, A., Mamiya, A. & Buzsáki, G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J. Neurosci. 19, RC20 (1999).
Schomburg, E. W. et al. Theta phase segregation of input-specific gamma patterns in entorhinal–hippocampal networks. Neuron 84, 470–485 (2014).
Watson, B. O., Levenstein, D., Greene, J. P., Gelinas, J. N. & Buzsáki, G. Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852 (2016).
Levenstein, D., Watson, B. O., Rinzel, J. & Buzsáki, G. Sleep regulation of the distribution of cortical firing rates. Curr. Opin. Neurobiol. 44, 34–42 (2017).
Chen, C. et al. Recent advances in electrochemical glucose biosensors: a review. RSC Advances 3, 4473–4491 (2013).
Basu, A. et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 62, 4083–4087 (2013).
Kovatchev, B. P., Shields, D. & Breton, M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol. Ther. 11, 139–143 (2009).
Baker, D. A. & Gough, D. A. Dynamic delay and maximal dynamic error in continuous biosensors. Anal. Chem. 68, 1292–1297 (1996).
Davey, R. J., Low, C., Jones, T. W. & Fournier, P. A. Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J. Diabetes Sci. Technol. 4, 1393–1399 (2010).
Keenan, D. B., Mastrototaro, J. J., Voskanyan, G. & Steil, G. M. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J. Diabetes Sci. Technol. 3, 1207–1214 (2009).
Donoghue, T. et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 23, 1655–1665 (2020).
Tingley, D. & Buzsáki, G. Transformation of a spatial map across the hippocampal–lateral septal circuit. Neuron 98, 1229–1242 (2018).
Stark, E. et al. Pyramidal cell–interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480 (2014).
Fernández-Ruiz, A. et al. Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019).
Acknowledgements
We thank M. Elmaleh, D. Nitz, A. Fernandez-Ruiz and A. Maurer for feedback on an early version of the manuscript. Funding was from NIH MH122391, U19 NS104590 and U19NS107616.
Author information
Authors and Affiliations
Contributions
D.T. and G.B. conceived the experiments. D.T., J.C., K.M. and E.K. performed the experiments presented. D.T. analysed data. D.T. and G.B. wrote the manuscript with contributions from other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks H. Freyja Olafsdottir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Continuous glucose monitor quality control, circadian, mealtime and spectral properties.
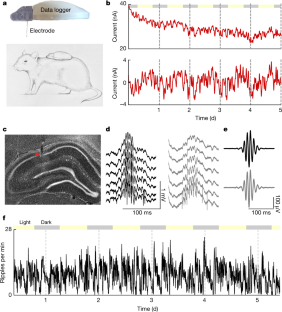
a, For a subset of experiments (n = 43 sessions in 8 rats) in which a continuous glucose monitor was implanted, the raw data are shown. Sorted by duration of patency. b, Average current readings (y axis) over time (x axis) after implantation. Note the slow decay over days, an indication of glucose oxidase enzyme breakdown on the electrode surface. c, Derivative (Δ current) of the data shown in a, showing faster time-scale fluctuations in glucose concentrations. d, Absolute value of the average Δ current (y axis) over time (x axis) after implantation. Note the relatively stable readings over days, and that the slow decay in absolute current readings is no longer present. e, Interstitial glucose concentrations are modulated over the circadian timescale. f, Interstitial glucose concentrations increase during the postprandial period (n = 36 meals, collected from 4 rats). Meal size was one 3-g pellet of standard chow. g, Average power spectral density plot across rats (n = 8) of the Δ current signal. Red line is a linear best fit to the data. h, Subtraction of the linear fit in c from the observed power at each frequency band. Note the elevated power in the 15-min to 90-min range. Note also that the ultradian cycling of SPW-R and glucose levels are different (compare with Extended Data Fig. 3). i, Average auto-correlogram of glucose fluctuations across eight rats. Red dashed lines are the 99% confidence interval for each rat.
Extended Data Fig. 2 SPW-R detection quality control.
a, Auto-correlograms of all inter-ripple intervals. Each row is data from an individual rat. Note the peaks in the histogram from bursts of ripples, and the drop to zero, representing an approximately 20-ms refractory period between ripples. b, Heat map of the high-pass-filtered (>100 Hz) LFP for every SPW-R, sorted by peak amplitude of the event. c, Scatter plots of the z-scored ripple amplitude (x axis) and ripple frequency (y axis) for all ripples (black dots) and rats (rows).
Extended Data Fig. 3 Circadian and ultradian rhythms in SPW-R rate.
a, Histogram of SPW-R rate for data from eight rats and sessions (n = 1,568.5 recording hours from 8 rats; mean = 196 ± 84 h). b, Modulation of the SPW-R rate across the circadian timescale. Grey lines are individual rats, black line is the population average. c, Example auto-correlograms (±12 h) of SPW-R rate for three rats. d, Average power spectral density plot (n = 8 rats) of ripple rate. Note two peaks at about 24 h and about 4 h. e, Cross-correlograms of detected SPW-Rs and CA1 population firing rates at two temporal scales. Left, 100-ms bins; right, 60-s median filter. At slow temporal scales (>250 ms), CA1 SPW-Rs are associated with an overall decrease in CA1 firing rates.
Extended Data Fig. 4 SPW-R features and states that affect the correlation with glucose fluctuations.
a, Histograms of duration, amplitude, frequency and inter-ripple intervals across all detected SPW-Rs. b, Average effect of SPW-R features on peri-SPW-R glucose fluctuations. The y axes are time (±100 min) and the x axes are the percentile of each distribution (or time in milliseconds for ripple duration). White lines are the average effect at +10-min temporal offset. Red and blue asterisks indicate the column used for the plots in c. c, Red and blue traces highlight the effect on peri-SPW-R glucose fluctuations at two different positions in the distributions of ripple features in b. d, Cross-correlograms for four rats calculated on 4-h blocks of time in which the rat was fasted or fed. e, A sliding 2-h window of time was taken to calculate the cross-correlogram between SPW-Rs and glucose fluctuations before, during and after a 3-g meal was provided to fasted rats. f, The SPW-R–glucose fluctuation cross-correlogram was not different between fasting and fed states. g, Cross-correlograms for eight rats calculated using blocks of time in which the rat was in NREM sleep or awake. h, A sliding 2-h window of time was taken to calculate the cross-correlogram between SPW-Rs and glucose fluctuations, relative to brain state transitions. The negative correlation did not change in amplitude across either wake-to-NREM or NREM-to-wake state transitions. Bounds are one s.d. i, The SPW-R–glucose fluctuation cross-correlogram was not different between NREM and waking states. j, Average cross-correlograms between SPW-R rate and Δ current for 4-h windows centred at 06:00 h (blue), 12:00 h (red) and 20:00 (orange). k, Integrated cross-correlograms (5–20 min) over the 24-h light–dark cycle. l, Same line as in j, without s.d. bounds and zoomed in on the y axis. Note the cumulative effect size is only slightly larger during the dark period. Across rats, no hour of the day had a significantly different correlation than any other hour of the day (Student’s t-test; minimum P = 0.53). Note also that the circadian fluctuation of glucose level is not a mirror image of SPW-R rate (compare Extended Data Fig. 3b).
Extended Data Fig. 5 Reverse correlation and oscillatory coupling of glucose fluctuations with SPW-R rate.
a, For each experiment peaks and troughs in glucose fluctuations were identified (red or black dotted lines; >1 s.d.). b, The average rate of SPW-Rs is shown when triggered on peaks in the glucose derivative (black line) and troughs in the glucose derivative (red line). Shaded area shows three s.d. of a randomly circularly permuted null distribution (100 iterations). c, The average rate of stimulations is shown when triggered on peaks in the glucose derivative (black line) and troughs in the glucose derivative (red line). Shaded area, three s.d. of a randomly circularly permuted null distribution (100 iterations). d, Average power–power comodulogram at different frequencies of the glucose signal (y axis) and SPW-R rate signal (x axis) for 30 rats. Note that the patch of high correlations (R > 0.2) from 15–60 min is shifted upward, off of the diagonal. This indicates the SPW-R rate at slightly higher frequencies modulates glucose fluctuations at a slightly lower frequency, a hall-mark of phase-resetting mechanisms in biological systems. P < 1−24 using a Student’s t-test with circularly shuffled data. e, Top, phase–amplitude coupling for each rat when using the phase angle of the glucose fluctuation signal (x axis) and SPW-R rate as the amplitude (colour; each rat is z-scored). Bottom, average phase–amplitude coupling across all rats. P < 1−18 using the Rayleigh test for non-uniformity.
Extended Data Fig. 6 SPW-R rate is the best predictor of glucose concentration changes.
a, Leave-one-out prediction of glucose dynamics. Each heat map column represents the mean squared error difference between two models, one model with all predictors and a second with all predictors but one (leave-one-out analysis). The y axis is the temporal offset between predictors and glucose fluctuations (±100 min in 5-min increments). The held-out predictor for each column is labelled along the x axis. b, Mean squared error difference for each model pair at +10-min offset. Each grey dot represents a single model pair with a random train and test split of the data (100 iterations). Note the averages for each model pair are equivalent to the +10 y axis of a. Box represents median, and 25th and 75th percentiles. c, Pairwise comparison of models using different predictors. For each pair of models using different predictors, the mean squared error values were subtracted. Each bin colour indicates the average mean squared error difference across 100 iterations with random train and test splits. d, For each pairwise predictor comparison, a two-sample t-test was performed across the 100 iterations with different train and test splits. The number and colour in each bin indicate the number of rats for which the mean squared error for the predictor column was significantly (P < 0.05) lower than the mean squared error for the predictor row. For example, the upper leftmost value of 6 indicates that ripple rate was a better predictor than brain state in 6 of the 8 rats. e, Latent variables do not predict glucose fluctuations more strongly than SPW-R rate. The same pairwise approach was taken as in c, d, using a generalized linear model with two predictors to estimate glucose fluctuations. The predictors used were the principal components, or independent components, of all predictors listed in Fig. 3. SPW-R rate, by itself, was also included as an additional predictor. Columns are sorted by best prediction. For each pairwise comparison SPW-R rate allowed for better (or equivalent, for principal component 1) prediction. f, For each pair of models, the number of rats for which where one model performed significantly better (two-way t-test P < 0.05) is shown. SPW-R rate and PC1 were similar in all rats, all other principal components and independent components performed worse for at least 4 out of 8 rats.
Extended Data Fig. 7 Stimulation protocol, statistics of optogenetic stimulation effect and control PPC experiments.
a, Autocorrelation of stimulation protocol showing no ultradian structure. b, Average stimulation rate was not different across the circadian light–dark cycle. c, Average P value of correlation between stimulation rate and Δ current at different temporal offsets (x axis) across all 8 rats. d, Number of rats (8 total) with P < 0.05 correlations between stimulation rate and Δ current at different temporal offsets (x axis). All tests were two-way t-tests. e, Optogenetic induction of population bursts in posterior parietal cortex does not induce decreases in glucose. Top, example histology. Bottom, example histology from the same experiment conducted in the PPC in 5 rats. f, Top, stimulation effect from CA1 experiments (as in Fig. 4). Bottom, stimulation effect from the same stimulation protocol in PPC. g, Red line is the average cross-correlogram between CA1 artificial ripples and glucose fluctuations. Blue line is the average cross-correlogram between PPC artificial ripples and glucose fluctuations. h, P values from a two-way t-test when comparing the cross-correlograms from the CA1 and PPC cohorts of rats. i, For reference, the cross-correlogram between artificial ripples and interstitial glucose fluctuations are shown (average from 8 rats). j, Average cross-correlogram between artificial ripples and the power spectrum slope of the LFP recorded in CA1. k, Average cross-correlogram between artificial ripples and the high-pass-filtered coherence (a proxy for EMG42). l, Average cross-correlogram between artificial ripples and movement as detected by an accelerometer on the head of the rat. m, Average cross-correlogram between artificial ripples and the ratio of wake to NREM sleep in each 5-min bin.
Extended Data Fig. 8 Nonlinear rectified response of lateral septum neurons to hippocampal synchrony.
a, Dorsal CA1 and CA3 projections converge within the lateral septum. Top, injection sites. Bottom, anterograde projections to the lateral septum. b, Left, black trace is one second of raw LFP recorded from CA1 pyramidal layer during running on a maze. Each row in the black raster plot indicates the action potentials from a single neuron. Grey dashed lines indicate the separation of theta cycles. The numbers below indicate the number of cells participating, and number of action potentials occurring, during each theta cycle. Right, three example SPW-Rs during sleep from the same session. In all example traces, neurons are sorted by the ordering of place fields on the maze (same sorting as left). c, Top, the proportion of theta cycles (black) and SPW-Rs (red) as a function of the hippocampal synchrony distribution. Dashed black and red vertical lines mark the position along the population synchrony axis at which 75% of all theta cycles (from left) or 75% of all SPW-Rs (from right) occurred. Bottom, the average z-scored firing rate across the lateral septum population (minimum threshold of 15 neurons) is shown as a function of the percentile of hippocampal synchrony distribution (n = 38 recordings from 5 rats). Bounds are one s.d.
Extended Data Fig. 9 Dorsal CA1 SPW-Rs more strongly correlate with glucose fluctuations than do ventral CA1 SPW-Rs.
a, Example histological verification of dorsal and ventral CA1 recording sites. Red triangles indicate the approximate location of the CA1 SPW-R detection channels for each region. Grey triangle indicates dorsal CA3. b, Example traces of raw LFP and bandpass-filtered LFP across both recording sites (top, ventral; bottom, dorsal). For each region, one shank with uniformly distributed recording sites along the dorsal–ventral axis is shown. c, Example LFP from channels used for ripple detection. d, Average cross-correlogram between dorsal CA1 and ventral CA1 SPW-Rs at slow timescales. Inset, average cross-correlogram between dorsal CA1 and ventral CA1 SPW-Rs at fast timescales. e, Across the three rats with simultaneous dorsal and ventral hippocampal recordings, dorsal SPW-Rs were equivalently correlated with glucose fluctuations as the previous cohorts of rats with only dorsal CA1 recordings (n = 30). Ventral SPW-Rs had a significantly (two-way t-test) weaker correlation with peripheral glucose fluctuations. f, For each rat, ventral CA1 SPW-Rs were more weakly correlated with glucose dynamics and dorsal CA1 SPW-Rs.
Extended Data Fig. 10 Histology from DREADD and CNO experiments.
a, Top, histology from the cohort of rats with AAV1-hDlx-GiDREADD-dTomato-Fishell injected into lateral septum. Bottom, example cross-correlograms between SPW-R and glucose fluctuations on vehicle (blue) and CNO (black) days. b, Top, histology from the cohort of rats with AAV2-hSyn-hM4D(Gi)-mCherry injected into medial septum. Bottom, example cross-correlograms between SPW-R and glucose fluctuations on vehicle (blue) and CNO (black) days. c, Comparison of effects for individual rats across vehicle versus CNO days for each cohort. d, P values (two-way t-test) for each part of the cross-correlograms shown in e when comparing within hM4Di rats (red; CNO versus vehicle) or across cohorts of rats (blue; CNO–hM4Di vs CNO–control). e, Ten colleagues who were not involved with these experiments were provided histology from all 9 rats (6 lateral septum; 3 medial septum) and asked to rate the degree of viral transfection in each subregion of the septum. Each rat had three images taken along the anterior–posterior axis and the individuals were blinded as to which virus had been injected and the effects of CNO on the SPW-R–glucose correlation. For each rat and subregion, the average score from these 10 individuals is shown in the table. For each subregion, the correlation between these scores and the effect of CNO was calculated and is shown in bold below. Red values had a P values < 0.05. f, Scatter plot of the SPW-R–glucose correlation after CNO injection (x axis) and the average expression score given by these rates (y axis) for the dorsal lateral septum. g, Scatter plot of the SPW-R–glucose correlation after CNO injection (x axis) and the average expression score given by these rates (y axis) for the medial septum.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, the Supplementary Discussion and Supplementary References.
Rights and permissions
About this article
Cite this article
Tingley, D., McClain, K., Kaya, E. et al. A metabolic function of the hippocampal sharp wave-ripple. Nature 597, 82–86 (2021). https://doi.org/10.1038/s41586-021-03811-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03811-w
This article is cited by
-
Interneuronal GluK1 kainate receptors control maturation of GABAergic transmission and network synchrony in the hippocampus
Molecular Brain (2023)
-
A brain cytokine-independent switch in cortical activity marks the onset of sickness behavior triggered by acute peripheral inflammation
Journal of Neuroinflammation (2023)
-
Spectro-spatial features in distributed human intracranial activity proactively encode peripheral metabolic activity
Nature Communications (2023)
-
Respiratory entrainment of units in the mouse parietal cortex depends on vigilance state
Pflügers Archiv - European Journal of Physiology (2023)
-
Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure
Molecular Psychiatry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.