Abstract
In early mitosis, the duplicated chromosomes are held together by the ring-shaped cohesin complex1. Separation of chromosomes during anaphase is triggered by separase—a large cysteine endopeptidase that cleaves the cohesin subunit SCC1 (also known as RAD212,3,4). Separase is activated by degradation of its inhibitors, securin5 and cyclin B6, but the molecular mechanisms of separase regulation are not clear. Here we used cryogenic electron microscopy to determine the structures of human separase in complex with either securin or CDK1–cyclin B1–CKS1. In both complexes, separase is inhibited by pseudosubstrate motifs that block substrate binding at the catalytic site and at nearby docking sites. As in Caenorhabditis elegans7 and yeast8, human securin contains its own pseudosubstrate motifs. By contrast, CDK1–cyclin B1 inhibits separase by deploying pseudosubstrate motifs from intrinsically disordered loops in separase itself. One autoinhibitory loop is oriented by CDK1–cyclin B1 to block the catalytic sites of both separase and CDK19,10. Another autoinhibitory loop blocks substrate docking in a cleft adjacent to the separase catalytic site. A third separase loop contains a phosphoserine6 that promotes complex assembly by binding to a conserved phosphate-binding pocket in cyclin B1. Our study reveals the diverse array of mechanisms by which securin and CDK1–cyclin B1 bind and inhibit separase, providing the molecular basis for the robust control of chromosome segregation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under accession codes EMD-12368 and EMD-12369 for the separase–CCC and separase–securin complexes, respectively. Protein coordinates for separase–CCC and separase–securin have been deposited in the Protein Data Bank (PDB) under accession codes 7NJ0 and 7NJ1, respectively. Source data are provided with this paper.
Change history
30 July 2021
This Article was amended to correct the linking to Supplementary Videos 1-3
References
Gruber, S., Haering, C. H. & Nasmyth, K. Chromosomal cohesin forms a ring. Cell 112, 765–777 (2003).
Uhlmann, F., Wernic, D., Poupart, M. A., Koonin, E. V. & Nasmyth, K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375–386 (2000).
Hauf, S., Waizenegger, I. C. & Peters, J. M. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293, 1320–1323 (2001).
Waizenegger, I. C., Hauf, S., Meinke, A. & Peters, J. M. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103, 399–410 (2000).
Ciosk, R. et al. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93, 1067–1076 (1998).
Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P. & Kirschner, M. W. Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 (2001).
Boland, A. et al. Cryo-EM structure of a metazoan separase-securin complex at near-atomic resolution. Nat. Struct. Mol. Biol. 24, 414–418 (2017).
Luo, S. & Tong, L. Molecular mechanism for the regulation of yeast separase by securin. Nature 542, 255–259 (2017).
Gorr, I. H. et al. Essential CDK1-inhibitory role for separase during meiosis I in vertebrate oocytes. Nat. Cell Biol. 8, 1035–1037 (2006).
Gorr, I. H., Boos, D. & Stemmann, O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19, 135–141 (2005).
Santaguida, S. & Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485 (2015).
Kamenz, J. & Hauf, S. Time to split up: dynamics of chromosome separation. Trends Cell Biol. 27, 42–54 (2017).
Rosen, L. E. et al. Cohesin cleavage by separase is enhanced by a substrate motif distinct from the cleavage site. Nat. Commun. 10, 5189 (2019).
Zou, H., McGarry, T. J., Bernal, T. & Kirschner, M. W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418–422 (1999).
Li, J., Ouyang, Y. C., Zhang, C. H., Qian, W. P. & Sun, Q. Y. The cyclin B2/CDK1 complex inhibits separase activity in mouse oocyte meiosis I. Development 146, dev182519 (2019).
Hellmuth, S., Gómez-H, L., Pendás, A. M. & Stemmann, O. Securin-independent regulation of separase by checkpoint-induced shugoshin–MAD2. Nature 580, 536–541 (2020).
Hellmuth, S. et al. Human chromosome segregation involves multi-layered regulation of separase by the peptidyl-prolyl-isomerase Pin1. Mol. Cell 58, 495–506 (2015).
Shindo, N., Kumada, K. & Hirota, T. Separase sensor reveals dual roles for separase coordinating cohesin cleavage and cdk1 inhibition. Dev. Cell 23, 112–123 (2012).
Lin, Z., Luo, X. & Yu, H. Structural basis of cohesin cleavage by separase. Nature 532, 131–134 (2016).
Sullivan, M., Hornig, N. C. D., Porstmann, T. & Uhlmann, F. Studies on substrate recognition by the budding yeast separase. J. Biol. Chem. 279, 1191–1196 (2004).
Nagao, K. & Yanagida, M. Securin can have a separase cleavage site by substitution mutations in the domain required for stabilization and inhibition of separase. Genes Cells 11, 247–260 (2006).
Alexandru, G., Uhlmann, F., Mechtler, K., Poupart, M. A. & Nasmyth, K. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105, 459–472 (2001).
Boos, D., Kuffer, C., Lenobel, R., Körner, R. & Stemmann, O. Phosphorylation-dependent binding of cyclin B1 to a Cdc6-like domain of human separase. J. Biol. Chem. 283, 816–823 (2008).
Kõivomägi, M. et al. Multisite phosphorylation networks as signal processors for Cdk1. Nat. Struct. Mol. Biol. 20, 1415–1424 (2013).
McGrath, D. A. et al. Cks confers specificity to phosphorylation-dependent CDK signaling pathways. Nat. Struct. Mol. Biol. 20, 1407–1414 (2013).
Holland, A. J., Böttger, F., Stemmann, O. & Taylor, S. S. Protein phosphatase 2A and separase form a complex regulated by separase autocleavage. J. Biol. Chem. 282, 24623–24632 (2007).
Zou, H., Stemman, O., Anderson, J. S., Mann, M. & Kirschner, M. W. Anaphase specific auto-cleavage of separase. FEBS Lett. 528, 246–250 (2002).
Luo, S. & Tong, L. in Macromolecular Protein Complexes III: Structure and Function. (eds. Harris, J. R. & Marles-Wright, J.) 217–232 (Springer, 2021).
Bachmann, G. et al. A closed conformation of the Caenorhabditis elegans separase-securin complex. Open Biol. 6, 160032 (2016).
Dephoure, N. et al. A quantitative atlas of mitotic phosphorylation. Proc. Natl Acad. Sci. USA 105, 10762–10767 (2008).
Cheng, K. Y. et al. The role of the phospho-CDK2/cyclin A recruitment site in substrate recognition. J. Biol. Chem. 281, 23167–23179 (2006).
Russo, A. A., Jeffrey, P. D., Patten, A. K., Massagué, J. & Pavletich, N. P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382, 325–331 (1996).
Melesse, M., Bembenek, J. N. & Zhulin, I. B. Conservation of the separase regulatory domain. Biol. Direct 13, 7 (2018).
Goda, T., Ishii, T., Nakajo, N., Sagata, N. & Kobayashi, H. The RRASK motif in Xenopus cyclin B2 is required for the substrate recognition of Cdc25C by the cyclin B-Cdc2 complex. J. Biol. Chem. 278, 19032–19037 (2003).
Zhang, Z., Yang, J. & Barford, D. Recombinant expression and reconstitution of multiprotein complexes by the USER cloning method in the insect cell-baculovirus expression system. Methods 95, 13–25 (2016).
Zhang, S. et al. Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature 533, 260–264 (2016).
Martin, T. G., Boland, A., Fitzpatrick, A. W. P. & Scheres, S. H. W. Graphene Oxide Grid Preparation. https://figshare.com/articles/Graphene_Oxide_Grid_Preparation/3178669 (2016).
Stabrin, M. et al. TranSPHIRE: automated and feedback-optimized on-the-fly processing for cryo-EM. Nat. Commun. 11, 5716 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Yang, Z., Fang, J., Chittuluru, J., Asturias, F. J. & Penczek, P. A. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure 20, 237–247 (2012).
Wilkinson, M. E., Kumar, A. & Casañal, A. Methods for merging data sets in electron cryo-microscopy. Acta Crystallogr. D 75, 782–791 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Preprint at https://doi.org/10.1101/2020.06.12.148296 (2020).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Brown, N. R. et al. CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat. Commun. 6, 6769 (2015).
Topf, M. et al. Protein structure fitting and refinement guided by cryo-EM density. Structure 16, 295–307 (2008).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D 74, 814–840 (2018).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Yang, Z. et al. UCSF Chimera, MODELLER, and IMP: an integrated modeling system. J. Struct. Biol. 179, 269–278 (2012).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We thank Y. Pfister and I. Flückinger for technical assistance; A. K. Höfler and L. Poulain for input and discussion; J. Kamenz, R. Loewith and F. Steiner for critical reading of the manuscript; J. Yang and Z. Zhang for assistance in the early stages of this project; O. Hofnagel and D. Prumbaum for assistance with EM data collection at the Max Planck Institute of Molecular Physiology; C. Alfieri for sharing his PLK1 plasmid; the computing department of the University of Geneva for providing an infrastructure to perform cryo-EM analysis; N. Roggli for maintaining computing in the Molecular Biology department; C. Bauer for his contributions to the cryo-EM facility in Geneva (cryoGEnic); O. Barabas for critical input; and the Metabolomics Core Facility at EMBL Heidelberg for mass spectrometry analysis. We acknowledge Diamond for access and support of the cryo-EM facilities at the UK national electron bio-imaging centre (eBIC), proposal EM13708, funded by the Wellcome Trust, MRC and BBSRC. This work was supported by the Swiss National Science Foundation (310030_185235), the Schmidheiny Foundation, a Novartis Research grant (all awarded to A.B.), a grant from the US National Institute of General Medical Sciences (R35-GM118053) to D.O.M., and an MRC grant (MC_UP_1201/6) to D.B.
Author information
Authors and Affiliations
Contributions
J.Y. expressed and purified the securin(∆160)–separase complexes, the MBP–securin fusions and the CDK1–cyclin B1–CKS1 complexes. C.M.G. purified securin(∆138)–separase fusion protein. P.R. and C.M.G. performed separase cleavage activity assays. J.Y. and Y.S. prepared grids and T.R. collected EM data with contributions from S.C. and A.B. J.Y. and P.R. analysed EM data and J.Y. determined the 3D reconstructions. J.Y. and Y.S. built the model ab initio and P.R. made the figures. A.B. directed the project and designed experiments together with D.O.M. A.B. and D.O.M. wrote the manuscript with contributions and discussions from J.Y., P.R., C.M.G., T.R., Y.S., P.M.S., D.B. and S.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Silke Hauf, Frank Uhlmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Preparations and EM images of the human separase–securin and separase–CCC complexes.
a, SDS–PAGE gels of wild-type Hs separase–securin and Hs securin(∆160)–separase(C2029S)–CDK1–cyclin B1–CKS1. b, Representative cryo-electron micrographs of Hs separase–securin (left), Hs securin(∆160)–separase(C2029S)–CCC (middle), and Hs securin(∆138)–separase(C2029S) (right) collected on graphene oxide-coated EM grids. Scale bars, 500 Å. c, Gallery of two-dimensional class averages of Hs separase–securin (left), Hs securin(∆160)–separase(C2029S)–CCC (middle) and Hs securin(∆138)-separase(C2029S) (right) showing typical classes. White arrow, flexible N-terminal HEAT-repeat domain. Scale bars, 100 Å. To increase the number of views of separase in complex with either securin or the CCC complex, we used graphene oxide-coated electron microscopy (EM) grids as described7,37. We used the deep-learning software packages TOPAZ45 and crYOLO41 to establish reliable particle picking conditions that allowed the identification of rare particle projections. d, Gold standard FSC curve for full-length separase–ecurin, focused refined separase–securin C-terminal domains (TPR-like and protease domains) and securin(∆160)–separase(C2029S)–CCC complexes. e, EM density maps colour-coded according to local resolution ranging from 2.8 Å to 6 Å
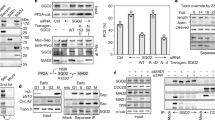
Extended Data Fig. 2 Evaluation of complex formation by size-exclusion chromatography (SEC).
a, SEC runs on the isolated PIN1, securin(∆160)–separase(C2029S) and CDK1–cyclin B1–CKS1 complexes resulted in elution volumes of approximately 1.9 ml, 1.5 ml and 1.6 ml, respectively (green, blue and red dashed lines). Adding the CDK1–cyclin B1–CKS1 complex (CCC) to securin(∆160)–separase(C2029S) did not yield a stable interaction between these two complexes under these conditions (solid red line). Adding PIN1 to the securin(∆160)–separase(C2029S) complex in the presence of CCC did not result in a detectable interaction of these two complexes (solid yellow line). However, a shoulder at high molecular weight can be observed, indicating partial phosphorylation in insect cells, in accordance with our mass spectrometry results in c. Adding ATP to securin(∆160)–separase(C2029S), PIN1 and CCC resulted in a clear shift to a 1.35-ml elution volume (solid purple line). A clear shift was also observed when we added ATP to securin(∆160)–separase(C2029S) and CCC, indicating that PIN1 is dispensable for complex formation in vitro (solid blue line). b, Coomassie blue-stained polyacrylamide gels of fractions from the SEC runs. c, Mass spectrometry analysis of human separase, without the CCC complex, shows phosphorylation of numerous sites in separase, including Ser1126. Each vertical bar indicates the number of times a peptide containing that site was identified, coloured to indicate the presence of phosphorylation: for Ser1126, blue segments indicate high-confidence assignment of phosphorylation (Mascot peptide score >32) at three peptides, and red segments indicate lower scores at two peptides. The colour of the amino acid label indicates the Mascot maximum delta mod score; a score of 10 for Ser1126 indicates high-confidence assignment of phosphorylation to that residue. d, Mass spectrometry analysis of human separase after incubation with 5 mM ATP, 10 mM Mg2+ and the CCC complex also shows phosphorylation of separase Ser1126, with three high-confidence peptides (score >32) and a maximum delta mod score of 8, also indicating a high-confidence assignment. Note that this analysis does not allow a rigorous quantitative comparison of Ser1126 phosphorylation in the two preparations
Extended Data Fig. 3 Representative EM density for ab initio model building.
a, Securin density at different threshold levels (stronger information at the N terminus of securin). Side-chain density is clearly visible. b, Density of AIL1 located in a cleft between the TPR-like and protease domains (left) and the CDC6-like domain of insert 2 (right). EM density allows the unambiguous placement of side chains in both loop segments. c, Density of the TPR-like domain of human separase. d, Examples of EM map quality for the separase protease domain. e, Strong density of phosphothreonine 161 in CDK1 (left) and the separase phosphoserine 1126 of the cyclin B1-binding loop (right). f, Extra density of human CKS1, possibly due to binding of phosphorylated threonine 1346 of separase.
Extended Data Fig. 4 Data-processing flowchart for the two datasets of the human separase–securin complex.
See Methods. 3D classification of separase–securin complexes resulted in about 55% of particles with strong density for the N-terminal HEAT-repeat domain. These particles were subjected to CTF refinement and classified on either the N or C terminus. We used particle subtraction for further classification. Classification on the N-terminal domain was executed without alignment (in contrast to the larger C-terminal part) and resulted in approximately 200,000 particles that were used for a final 3D reconstruction. In both cases, DeepEMhancer47 was used for post-processing.
Extended Data Fig. 5 Data-processing flowchart for the human separase–CCC complex.
See Methods. 3D classification of securin(∆160)–separase(C2029S)–CCC complexes resulted in approximately 312,000 particles subjected to CTF refinement and Bayesian polishing. The final reconstruction refined to roughly 3.6 Å resolution and was post-processed in RELION.
Extended Data Fig. 6 Comparison of flexible elements in separase proteins.
a, Comparison of human separase in complex with securin or CCC to budding yeast (Sc) and C. elegans (Ce) separase. The loops—AIL1, AIL2, AIL3 (highlighted in blue) and the cyclin B-binding loop (highlighted in purple)—become ordered upon binding of CCC. The N-terminal domain adopts an elongated shape in human separase (two left structures) but in yeast this N-terminal domain is kinked. This domain is lost in C. elegans separase. b, Comparison of the N-terminal domains of human and yeast separases, with EM density for the first approximately 250 residues of human separase indicated as blue cartoon. The yeast N-terminal domain adopts a much more compact fold, with the N terminus of the yeast protein folding back in close proximity to the TPR-like domain. This folded architecture may be an intrinsic feature of the yeast N-terminal domain or due to crystal packing constraints. c, EM density of human separase–securin at low-threshold rendering allows a full representation of the enzyme (grey envelope). A predicted model of the N-terminal 250 residues is shown in yellow, and the EM-derived structure of separase–securin is shown in blue and orange, respectively.
Extended Data Fig. 7 Multiple sequence alignments of conserved docking motifs in SCC1, securin and AILs of separase.
a, Sequence comparison of the catalytic site binding motif in SCC1 (purple), securin (orange) and the AIL3 of separase (grey) in different species. Key residues are boxed. The invariant arginine residue involved in substrate catalysis (SCC1) is replaced by a large hydrophobic residue in securin. b, Sequence comparison of the NXLXΦE binding motif in SCC1, securin and AIL1 of separase. A phenylalanine three amino acids upstream of the docking motif might correspond to the P1 position in SCC1 (arginine) or securin (hydrophobic residue). c, Sequence comparison of the LPE docking motif in SCC1 and securin. d, Residues in securin and AIL2 of separase that bind to a hydrophobic cleft situated in the TPR-like domain of separase. See also Extended Data Fig. 11a.
Extended Data Fig. 8 Molecular surface charge representation of the separase–securin and separase–CDK1–cyclin B1 complexes.
Surface charge of separase reveals a basic groove near the catalytic site that facilitates binding of AIL3 (top) and securin (bottom). Note that AIL3 binds in an inverted orientation to separase when compared to SCC1 or securin. SCC1 phosphorylation at a nearby serine residue stimulates binding to this basic groove in yeast separase23. Phosphorylation of serine residues in AIL3 has been detected in other studies19,30. Sequence alignment (below) of the separase protease domain reveals conservation of residues that are critical for substrate or inhibitor recognition (boxed in grey).
Extended Data Fig. 9 SCC1 cleavage is enhanced by the NHLEYE motif downstream of the cleavage site.
a, SCC1 deletion mutants (left gel) were constructed to test the function of the 82-amino-acid region between the cleavage site (169EIMR) and the 255LPE docking site. Constructs were made in an internal SCC1 fragment (residues 142–300). Each construct removed from 22 to 82 residues around the centre of the intervening region, as indicated in the diagrams. In another series of constructs (right gel), different amounts of the intervening sequence were replaced with random linker sequence (G, S, A, and T). 35S-labelled SCC1 fragments were incubated with or without separase, and reaction products were analysed by SDS–PAGE and phosphorimaging. The central 22-amino acid region contains the conserved 207NHLEYE sequence. Results are representative of four independent experiments. b, The indicated amino acids were replaced with alanine in SCC1 (residues 142–300). 35S-labelled SCC1 mutants were incubated with or without separase, and reaction products were analysed by SDS–PAGE and phosphorimaging. Results are representative of four independent experiments. c, In SCC1, 30–40-residue spacers separate the cleavage site (169EIMR) and two docking sites (207NHLEYE and 255LPE). The importance of the spacer regions was tested with various mutations in an internal SCC1 fragment (residues 90–300). In the SCC1(∆S) mutant, both spacers were deleted. In the three linker mutants, the two intervening spacers were replaced, together or one at a time, with random linker sequence (G, S, A, and T). Results are representative of four independent experiments. The reaction on the far right demonstrates cleavage of the securin(RE) mutant (residues 93–150), in which the pseudosubstrate motif 115EKFFP is converted to EKFRE13. Thus, closely spaced docking motifs allow cleavage of securin but not of SCC1.
Extended Data Fig. 10 Loop deletions of AIL1 and AIL3 enhance separase cleavage activity.
a, Separase mutants carrying deletions of AIL1 and/or AIL3 were purified to analyse the effect of these loop segments on separase cleavage activity. The results are representative of three independent experiments. Regions deleted in each mutant as follows (see Extended Data Table 2 for sequence details): AIL1∆, deletion of AIL1, which harbours the NDLNYE motif; AIL3∆1, deletion of CDC6-like domain and NFS motif; AIL3∆2, deletion of entire insert 2; AIL3∆3, deletion of NFS motif. 35S-labelled full-length SCC1 was incubated with either wild-type (WT) separase, an inactive separase mutant (C2029S), or separase loop deletions as indicated. Reaction products were analysed by SDS–PAGE and phosphorimaging. All loop deletions show enhanced SCC1 cleavage activity, with the exception of a mutant carrying a deletion of the entire insert 2, which exhibits cleavage activity comparable to that of the wild-type protein. Quantification of three independent experiments shown in Fig. 2g. b, Full-length SCC1 cleavage assays as described in a in the presence or absence of the CCC complex. Results are representative of three independent experiments. While SCC1 cleavage by wild-type separase is fully repressed by the CCC complex (lane 3), mutant proteins with deletions of AIL1 and/or AIL3 show partial cleavage activity in the presence of the CCC complex. Deletion of insert 2 (AIL3∆2) leads to lower cleavage activity compared to other loop deletions. The reduction in inhibition by the CCC complex can be partially explained through reduced complex affinity, as demonstrated by SEC runs in c. Only wild-type separase (solid blue line) shows a clear shift towards a higher molecular weight elution volume after incubation with 5 mM ATP, 10 mM Mg2+ and the CCC complex. d, Schematic diagram of the loop mutants analysed in these experiments
Extended Data Fig. 11 A hydrophobic cleft in separase promotes securin binding and correlates directly with substrate cleavage efficiency.
a, Close-up of the binding interface of the hydrophobic cleft of separase interacting with securin (top left, orange) or the AIL2 of separase (top right, grey). The molecular surface representation (below) highlights the hydrophobic nature of the binding cleft in separase. b, Schematic representation of constructs used in binding and cleavage assays. c, In vitro pull-down assays using maltose binding protein (MBP)–securin fusions as bait demonstrates the importance of a stretch of hydrophobic amino acids in securin. A construct including residues 1–157 or wild-type securin (1–202) can bind to separase (lanes 9 and 10), whereas two C-terminal truncations (securin(∆127) and securin(∆138)) do not bind separase under these conditions (lanes 7 and 8). Purified MBP serves as negative control (lane 6). Lanes 1–5 are 10% of the input. Pull-down experiments have been repeated in three independent experiments. d, 35S-labelled full-length SCC1 proteins were incubated with or without separase, and reaction products were analysed by SDS–PAGE and phosphorimaging. SCC1 cleavage by separase is inhibited by addition of full-length securin 1–202 or truncated securin 1–157 (lanes 5 and 6). Shorter securin constructs (1–127 and 1–138), which lack a stretch of hydrophobic residues, do not inhibit cleavage under these conditions (lanes 3 and 4).
Extended Data Fig. 12 The CDK1–cyclin B1–CKS1 complex binds to a clearly defined cleft in separase.
a, The separase–securin complex has a clearly defined cleft between the TPR-like and protease domains. b, The separase–CCC complex in four orientations (each rotated by 90°). The HEAT-repeat domain is omitted for clarity.
Extended Data Fig. 13 Molecular surface charge representation of cyclins.
a, The cyclin B-binding loop of separase binds inverted to the substrate-docking hydrophobic patch of cyclin B1 when compared to common substrates. b, Sequence alignment of the cyclin B-binding loop docking site motif. c, Calculating the surface charge of A- and B-type cyclins illustrates the existence of a phosphate-binding pocket in cyclin B1 but not cyclin A (compare top left versus top right). The cyclin B-binding loop also binds to the substrate-docking hydrophobic patch of cyclin B1, comparable to a typical cyclin B1-binding partner. Multiple sequence alignment of residues lining the phosphate-binding pocket shows conservation of Arg307, His320 and Lys324 in B-type cyclins (bottom left) but not in cyclin A (bottom right).
Extended Data Fig. 14 Binding of separase to CDK1–cyclin B is mediated through two distinct loops.
a, Comparison of human CDK1 in a chemical inhibitor-bound state (green) and the separase–CCC complex (pale red) with the CDC6-like domain of separase (AIL3; cyan). In the separase–CCC complex, the activation loop is in its active conformation (downwards) and allows substrate and separase binding. In the inhibitor-bound structure, the activation loop conformation is incompatible with substrate binding. b, The CDC6-like domain of separase binds to the active site of CDK1, while the cyclin B-binding loop (purple) of separase wraps around cyclin B1 with pSer1126 in its centre and hydrophobic patch interactions nearby. In common cyclin B substrates, these interactions are mediated through one continuous polypeptide (red). c, A kink in AIL3 is formed at the interface of cyclin B1 and the separase protease domain, with Thr1389, Arg1390 and Leu1391 being key residues for binding of AIL3 to cyclin B1.
Supplementary information
Supplementary Figure 1
This file includes the uncropped gels and blots for Fig. 2f, and Extended Data Figs. 1a, 2b, 9a-c, 10a, b and 11c, d.
Video 1
Overall structure of inhibitory separase complexes This video shows the overall architecture of the human separase-securin and the separase- Cdk1-cyclin B1-Cks1 complexes as electron density and ribbon representation while rotating around the X-axis.
Video 2
Autoinhibitory loops mimic securin binding This video shows a superposition of the autoinhibitory loop 1 and 3 with securin and highlights common binding motifs that recognise structural elements in separase adjacent to the catalytic site.
Video 3
Separase and Cdk1-cyclin B1-Cks1 complex assembly This video describes the structural assembly of the separase-Cdk1-cyclin B1-Cks1 complex. The phosphate-binding pocket in cyclin B1 and the Cdc6-like domain are highlighted. A superposition between a Cdk1 structure bound to a substrate peptide and the separase Cdc6- like domain bound to the catalytic site of Cdk1 illustrates the structural similarities between these two binding partners.
Rights and permissions
About this article
Cite this article
Yu, J., Raia, P., Ghent, C.M. et al. Structural basis of human separase regulation by securin and CDK1–cyclin B1. Nature 596, 138–142 (2021). https://doi.org/10.1038/s41586-021-03764-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03764-0
This article is cited by
-
Genome-wide CRISPR screen identifies ESPL1 limits the response of gastric cancer cells to apatinib
Cancer Cell International (2024)
-
Kinesin Family Member-18A (KIF18A) Promotes Cell Proliferation and Metastasis in Hepatocellular Carcinoma
Digestive Diseases and Sciences (2024)
-
Cyclers’ kinases in cell division: from molecules to cancer therapy
Cell Death & Differentiation (2023)
-
Structural insights into the regulation of Cas7-11 by TPR-CHAT
Nature Structural & Molecular Biology (2023)
-
The structural flexibility of MAD1 facilitates the assembly of the Mitotic Checkpoint Complex
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.