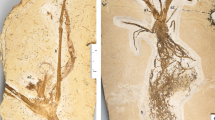
Abstract
The second integument of the angiosperm ovule is unique among seed plants, with developmental genetics that are distinct from those of the inner integument1. Understanding how the second integument should be compared to structures in other seed plants is therefore crucial to resolving the long-standing question of the origin of angiosperms2,3,4,5,6. Attention has focused on several extinct plants with recurved cupules that are reminiscent of the anatropous organization of the basic bitegmic ovules of angiosperms1,2,3,4,5,6, but interpretations have been hampered by inadequate information on the relevant fossils. Here we describe abundant exceptionally well-preserved recurved cupules from a newly discovered silicified peat dating to the Early Cretaceous epoch (around 125.6 million years ago) in Inner Mongolia, China. The new material, combined with re-examination of potentially related fossils, indicates that the recurved cupules of several groups of Mesozoic plants are all fundamentally comparable, and that their structure is consistent with the recurved form and development of the second integument in the bitegmic anatropous ovules of angiosperms. Recognition of these angiosperm relatives (angiophytes) provides a partial answer to the question of angiosperm origins, will help to focus future work on seed plant phylogenetics and has important implications for ideas on the origin of the angiosperm carpel.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data matrix for phylogenetic analyses is provided in Supplementary Data 1. Computed tomography data have been deposited in Dryad at https://doi.org/10.5061/dryad.5x69p8d2r.
Code availability
The MrBayes commands for Bayesian analysis are included in the NEXUS formatted file in Supplementary Data 2.
References
Gasser, C. S. & Skinner, D. J. Development and evolution of the unique ovules of flowering plants. Curr. Top. Dev. Biol. 131, 373–399 (2019).
Gaussen, H. Les Gymnospermes, Actuelles et Fossiles. Travaux du Laboratoire Forestier de Toulouse, Tome 2. Étud. Dendrol. Sect. 1, Vol. 1 (1946).
Stebbins, G. L. Flowering Plants: Evolution Above the Species Level (Harvard Univ. Press, 1974).
Doyle, J. A. Origin of angiosperms. Annu. Rev. Ecol. Syst. 9, 365–392 (1978).
Crane, P. R. Phylogenetic analysis of seed plants and the origin of angiosperms. Ann. Mo. Bot. Gard. 72, 716–793 (1985).
Frohlich, M. W. & Chase, M. W. After a dozen years of progress the origin of angiosperms is still a great mystery. Nature 450, 1184–1189 (2007).
Harris, T. M. A new member of the Caytoniales. New Phytol. 32, 97–113 (1933).
Harris, T. M. The Yorkshire Jurassic Flora, II. Caytoniales, Cycadales & Pteridosperms (British Museum, Natural History, 1964).
Hilton, J. & Bateman, R. M. Pteridosperms are the backbone of seed-plant phylogeny. J. Torrey Bot. Soc. 133, 119–168 (2006).
Doyle, J. A. Integrating molecular phylogenetic and paleobotanical evidence on origin of the flower. Int. J. Plant Sci. 169, 816–843 (2008).
Rothwell, G. W. & Stockey, R. A. Phylogenetic diversification of Early Cretaceous seed plants: the compound seed cone of Doylea tetrahedrasperma. Am. J. Bot. 103, 923–937 (2016).
Shi, G. et al. Diversity and homologies of corystosperm seed-bearing structures from the Early Cretaceous of Mongolia. J. Syst. Palaeontology 17, 997–1029 (2019).
Anderson, J. M. & Anderson, H. M. Heyday of the Gymnosperms: Systematics and Biodiversity of the Late Triassic Molteno Fructifications (Strelitzia, 2003).
Klavins, S. D., Taylor, T. N. & Taylor, E. L. Anatomy of Umkomasia (Corystospermales) from the Triassic of Antarctica. Am. J. Bot. 89, 664–676 (2002).
Axsmith, B. J., Taylor, E. L., Taylor, T. N. & Cuneo, N. R. New perspectives on the Mesozoic seed fern order Corystospermales based on attached organs from the Triassic of Antarctica. Am. J. Bot. 87, 757–768 (2000).
Taylor, T. N., Del Fueyo, G. M. & Taylor, E. L. Permineralized seed fern cupules from the Triassic of Antarctica: implications for cupule and carpel evolution. Am. J. Bot. 81, 666–677 (1994).
Bomfleur, B. et al. Habit and ecology of the Petriellales, an unusual group of seed plants from the Triassic of Gondwana. Int. J. Plant Sci. 175, 1062–1075 (2014).
Taylor, T. N. & Archangelsky, S. The Cretaceous pteridosperms Ruflorinia and Ktalenia and implications on cupule and carpel evolution. Am. J. Bot. 72, 1842–1853 (1985).
Friis, E. M. & Crane, P. R. & Pedersen, K. R. Geminispermum, an Early Cretaceous (early–middle Albian) cupulate unit from the angiosperm-dominated Puddledock flora of eastern North America. Acta Palaeobot. 59, 229–239 (2019).
Barbacka, M. & Boka, K. A new Early Liassic Caytoniales fructification from Hungary. Acta Palaeobot. 40, 85–111 (2000).
Crane, P. R. in Origin and Evolution of Gymnosperms (ed. Beck, C. B.) 218–272 (Columbia Univ. Press, 1988).
Endress, P. K. Angiosperm ovules: diversity, development, evolution. Ann. Bot. 107, 1465–1489 (2011).
Doyle, J. A. & Donoghue, M. J. Phylogenies and angiosperm diversification. Paleobiology 19, 141–167 (1993).
Blomenkemper, P., Kerp, H., Abu Hamad, A., DiMichele, W. A. & Bomfleur, B. A hidden cradle of plant evolution in Permian tropical lowlands. Science 362, 1414–1416 (2018).
Deng, S. Early Cretaceous Flora of Huolinhe Basin, Inner Mongolia, Northeast China (Geological Publishing House, 1995).
Shi, G. et al. Age of the Huolinhe Formation in the Huolinhe Basin, eastern Inner Mongolia, China: evidence from U–Pb zircon dating and palynological assemblages. J. Stratigr. 45, 69–81 (2021).
Crane, P. R. & Herendeen, P. S. Bennettitales from the Grisethorpe Bed (Middle Jurassic) at Cayton Bay, Yorkshire, UK. Am. J. Bot. 96, 284–295 (2009).
Huang, D. The Daohugou Biota (Shanghai Scientific & Technical Publishers, 2016).
Galtier, J. & Phillips, T. L. in Fossil Plants and Spores: Modern Techniques (eds Jones, T. P. & Rowe, N. P.) 67–70 (Geological Society of London, 1999).
Friis, E. M. et al. Phase-contrast X-ray microtomography links Cretaceous seeds with Gnetales and Bennettitales. Nature 450, 549–552 (2007).
Acknowledgements
We thank B. Zhang, C. Dong, S. Yin, S. Hu, H. Jiang, Q. Li, W. Zhang and F. Lu for assistance with fieldwork in Inner Mongolia, China; R. Serbet and B. Atkinson for assistance with examination and the loan of Petriellaea specimens from the University of Kansas collections; X. Wang and F. Zheng for the loan of the Caytonia fossils from the Daohugou Bed in the collections of the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences; Z.-X. Luo, A. I. Neander and C. Brodersen for assistance with micro-computed tomography (micro-CT) scanning; E.M. Friis, X. Xu and S. Donskaya for assistance in processing micro-CT data; H. Wang, C. Zhang and M. Carvalho for assistance with phylogenetic analyses; and D. Yang for assistance with drawing. This work was supported by the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2017359), the US National Science Foundation grant DEB-1748286, the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), the National Natural Science Foundation of China (41790454) and the Oak Spring Garden Foundation.
Author information
Authors and Affiliations
Contributions
P.R.C. and G.S. designed the research. G.S., F.H., P.S.H. and P.R.C. discovered the new Early Cretaceous silicified peat and collected the palaeobotanical samples. G.S., F.H. and E.G.C. prepared the fossil material and processed the micro-CT data. G.S., F.H., P.S.H. and P.R.C. analysed the data. G.S. and P.R.C. wrote the manuscript, in discussion with F.H., P.S.H. and E.G.C. P.R.C supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks William Friedman, Michael Frohlich and Douglas Soltis for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Fossil locality.
a, Map showing the location of the Zhahanaoer open-cast coal mine (indicated by a cupule) in Jarud Banner, eastern Inner Mongolia, China, and the Tevshiin Govi locality (left) in central Mongolia, where similar lignified cupules have been found12. b, Stratigraphic section of the ‘lower coal-bearing member’ of the Huolinhe Formation at the margin of the Zhahanaoer coal mine showing the position of the silicified plant fossils (indicated by a cupule) and dated ash layer26. c, Part of the outcrop showing the chert embedded in mudstones.
Extended Data Fig. 2 Fossil cupules and associated pollen from the Early Cretaceous of Inner Mongolia, China.
The small stems (j, m) and multiveined leaves (k) that occur with the cupules in the chert were probably produced by the same group of plants, but they remain to be linked conclusively. a, Individual seed-bearing unit that is attached to the axis of the cone in Fig. 1a. PB23663. b, Oblique transverse section of cupule showing the attachment of one seed (right) to the pad of parenchyma tissue, and the vascular bundle (VB) with concentrically arranged xylem. PB23673. c, Longitudinal section of cupule. PB23674. d, Line drawing of a showing the stalked cupule (blue) and bract (green). e, Line drawing of b showing the cupule (blue), seeds (yellow) with a single integument (brown), pad of parenchyma tissue (grey) where one seed is attached, and concentrically arranged xylem (orange). f, Line drawing of c. Note that the micropyle of seed (yellow) is oriented towards the base of the cupule (blue) stalk. g, h, Three-dimensional reconstructions from segmented micro-CT data. g, Individual seed-bearing unit that is attached to the axis of the cone in Fig. 3a, showing the bract (green), stalked cupule (blue) and seed (brown). PB23672. h, Three-angled seed that is attached to the cupule in g. i, Longitudinal section of a seed, showing the bifid micropyle (arrows), the nucellus (n), detached inner cuticle of integument (ic) and megaspore membrane (m). PB23675. j, Small stem with about 10 asymmetrical growth rings and well-developed pith (arrow). PB23676. k, Transverse section of a leaf showing nine longitudinal veins. PB23677. l, Bisaccate pollen grain adhering to the tip of nucellus within the integument. PB23678. m, Detail of secondary xylem of the small stem in j, showing uniseriate ray (arrow). Scale bars, 1 mm (a–c, g); 500 μm (h–j); 200 μm (k); 50 μm (m); 10 μm (l).
Extended Data Fig. 3 Details of vasculature at different levels in fossil cupules from the Early Cretaceous of Inner Mongolia, China.
The interpretative line drawings show the cupule (blue), xylem (orange) and poorly preserved phloem (dark blue). All sections are oriented in the same way as the vascular bundle in Fig. 2, with the upper side of each bundle towards the inner side of the cupule, and the lower side of each bundle towards the outer side of the cupule. a, Transverse section of the fused bract-cupule stalk complex (left) with line drawing (right), showing two median vascular bundles; the upper (adaxial) supplies the cupule and the lower (abaxial) supplies the bract. Note that in the two bundles the xylem (orange) is towards the centre of the complex relative to the spaces that indicate the former position of the phloem. PB23667. b, Detail of the upper vascular bundle in a. c, Transverse section of cupule stalk slightly distal to a (left) with line drawing (right), showing two abutting vascular bundles with the xylem (orange) towards the outer side of the cupule relative to the space that indicates the former position of the phloem. PB23667. d, Detail of the two abutting vascular bundles in c. e, Transverse section of cupule stalk near the base of the cupule (left) with line drawing (right), showing two vascular bundles with flattened xylem. PB23669. f, Detail of the left vascular bundle in e showing xylem (orange) and poorly preserved phloem (dark blue). g, Transverse section of cupule stalk near the apex of the cupule (left) with line drawing (right), showing two vascular bundles with inverted V-shaped xylem. PB23670. h, Detail of the right vascular bundle in g. i, Transverse section of cupule stalk near the tip of its strongly curved distal portion (left) with line drawing (right). PB23671. j, Detail of the left vascular bundle in i showing the circular outline of the xylem (orange) and poorly preserved phloem (dark blue). Scale bars, 100 μm (a, j); 50 μm (b, d, f, h); 200 μm (c, e), 500 μm (g, i).
Extended Data Fig. 4 Fossil cupules from the Early Cretaceous Tevshiin Govi Formation at the Tevshiin Govi locality in central Mongolia.
Light micrographs with line drawings showing the bract (green), stalked cupules (blue) and the axis on which they are borne (brown). a–f, Doylea mongolica (Umkomasia mongolica)12. a, Seed cone with lateral seed-bearing units loosely and helically arranged. PP56614. b, Line drawing of a. c, e, Upper (adaxial) (c) and lower (abaxial) (e) views of a lateral seed-bearing unit. PP55648. d, Line drawing of c. f, Line drawing of e. g–j, Umkomasia corniculata12. Upper (adaxial) (g) and lower (abaxial) (i) views of a lateral seed-bearing unit. PP56628. h, Line drawing of g. j, Line drawing of i. k–n, Umkomasia trilobata12. Upper (adaxial) (k) and lower (abaxial) (m) views of a lateral seed-bearing unit with a dorsiventrally flattened axis that bears the cupules. PP56684. l, Line drawing of k. n, Line drawing of m.
Extended Data Fig. 5 Mesozoic seed plants with recurved cupules.
a, Umkomasia quadripartita from the Late Triassic of South Africa; line drawing of the holotype. b, Kannaskoppia vincularis from the Late Triassic of South Africa; line drawing of the holotype. c, Reconstruction of Umkomasia quadripartita. d, Reconstruction of Kannaskoppia vincularis. The reconstructions in a–d are all based on a previous work13. e, Reconstruction of Umkomasia resinosa from the upper Middle or Upper Triassic of Antarctica14. f, Reconstruction of Umkomasia uniramia from the Late Triassic of Antarctica15. g, Reconstruction of a shoot bearing cupules of Ktalenia circularis and leaf of Ruflorinia sierra from the Early Cretaceous of Argentina18. h, Details of cupules of Ktalenia circularis that may contain one or two seeds18.
Extended Data Fig. 6 Fossil cupules and cupule stalks of Caytonia sp.
a–i, Caytonia sp. from the Middle Jurassic of Yorkshire, UK. a, b, Flattened cupule-bearing structures showing subopposite cupule stalks or attachment scars in two ranks that lack evidence of a bract subtending each cupule. a, PP60606. b, PP60607. c–e, Small, possibly aborted, cupules at pollination stage showing the distinct lips. c, PP60608. d, PP60609. e, Note the parallel grooves leading to the micropylar canals on the lip of the cupule. PP60610. f, Flattened seed removed from a cupule. PP60611. g, Fractured mature cupule showing seeds with the micropyles of the two on the left oriented towards the base of the cupule. PP60612. h, i, Caytonia nathorsti8. h, Flattened cupule-bearing structure showing lateral cupules recurved towards the presumed upper (adaxial) surface. V.26661 (Natural History Museum, London). i, Portion of h showing the ridges on the presumed lower (abaxial) surface. j–l, Caytonia sp. from the Middle–Late Jurassic of Daohugou locality, eastern Inner Mongolia, China. j, k, Part and counterpart of a cupule containing eleven seeds arranged in two rows. See Fig. 3g for a three-dimensional reconstruction from segmented micro-CT data. B0441. l, Cupule showing the parallel grooves leading to the micropylar canals (arrow). B0440 (IVPP). Scale bars, 500 μm (a, c–g); 1 mm (b, h–l).
Extended Data Fig. 7 Permineralized cupules from the Triassic of Antarctica.
All specimens are deposited in the palaeobotanical collections of the University of Kansas. The interpretative line drawings show cupule (blue) and seed (yellow) with a single integument (brown). a–k, Petriellaea triangulata. a, Longitudinal section of empty seed-bearing structures. Note possible bract (arrow) fused to the cupule stalk. 10852A. b, Line drawing of a showing possible bract (stippled green). c, Transverse section of a cupule containing five seeds. Note the possible bract (arrow). 10025G. d, Line drawing of c showing the possible bract (stippled green). e, Transverse section of a cupule. 10023A. f, Line drawing of e. g–j, Detail of the vascular bundles of the cupule stalk, oriented in the same way as the vascular bundle in Fig. 2, Extended Data Fig. 3, with the upper side of each bundle towards the inner side of the cupule, and the lower side of each bundle towards the outer side of the cupule. 10025G. h, Line drawing of the vascular bundle in g showing the xylem (orange) that appears arranged in a circle in transverse section. j, Line drawing of the vascular bundle in i showing the xylem (orange). Note that in g, i there are no obvious sieve elements or space that may represent the former position of the phloem towards the outer side of the cupule (lower side of the image) relative to the xylem as originally interpreted16. k, Three-dimensional reconstruction from segmented micro-CT data showing three-angled seed in lateral and apical views. 10025G. l–o, Umkomasia resinosa. Transverse sections of cupules from the type material with interpretive line drawings. 11323. l, Cupule formed by the cupule stalk and two lateral flaps and containing two seeds. m, Line drawing of l. n, Cupule formed by the cupule stalk and flap and containing a single seed. o, Line drawing of n. Scale bars, 1 mm (a, l, n); 500 μm (c, e, k); 50 μm (g–j).
Extended Data Fig. 8 Interpretive diagrams of seed-bearing structures of Mesozoic plants with recurved cupules, with longitudinal sections of Geminispermum virginiense and an idealized angiosperm carpel.
The interpretative diagrams show the bract (green), the stalked cupules (blue) and the axis on which they are borne (red–brown), and seed consisting of the nucellus (yellow) with a single integument (brown). a, Fossil cupules from the Early Cretaceous of Inner Mongolia, China. The cupules are borne helically on a central axis, each cupule contains two seeds, and is subtended by a bract. b, Doylea11,12. Each lateral seed-bearing unit consists of a bract subtending an axis that bifurcates into two cupule stalks; each cupule stalk bears one cupule that contains a single seed. Individual cupules are not subtended by a bract. c, Umkomasia trilobata12. Each lateral seed-bearing unit consists of a bract subtending a flattened axis that is divided distally into three flattened cupule stalks; each cupule stalk bears one cupule that contains a single seed. Individual cupules are not subtended by a bract. d, Umkomasia resinosa14. Cupules are borne helically on a branched elaborated axis and each cupule contains one or two seeds. The presence of a bract is not confirmed but the whole structure may have been borne in the axil of a bract or leaf. Individual cupules are not subtended by a bract. e, Umkomasia quadripartita13. The cupules are borne more or less decussately on an axis with two pairs of bracts near the base; each cupule contains a single seed. Individual cupules are not subtended by a bract. f, Caytonia7,8. The cupules are subopposite in two ranks on an elaborated flattened axis; each cupule contains up to 30 seeds arranged in two or more rows. There are no bracts subtending individual cupules and the presence of a bract subtending the whole structure is not confirmed. g, Petriellaea triangulata16 and Kannaskoppia vincularis13. The cupules are probably borne helically (P. triangulata) or in two ranks (K. vincularis) on an axis; each cupule contains several seeds arranged in one row. Interpretation in which individual cupules are not subtended by bract. h, Alternative interpretation of the seed-bearing structure of P. triangulata, in which each cupule is subtended by a bract as the Inner Mongolia cupules in a. i, Umkomasia uniramia15. Stalked cupules are borne in a whorl of four to eight at the tip of an axis bearing a pair of bracts, each cupule contains a single seed. j, k, Geminispermum virginiense19. j, A pair of recurved cupules face each other at the tip of an axis. k, Each cupule contains a single seed and is subtended by a bract. l, Angiosperm carpel with a bitegmic ovule curved in the same direction of the curvature of the enclosing carpel (syntropous)22, showing the carpel (green), funiculus and second integument (blue), and inner integument (brown) and nucellus (yellow).
Extended Data Fig. 9 Phylogenetic relationships of seed plants based on parsimony, maximum likelihood and Bayesian analyses of a dataset for living and fossil seed plants.
See Supplementary Data 1 for the dataset. All trees are rooted with Elkinsia. As with all previous analyses of seed plant relationships5,9,10,30 support for most nodes is not strong. a, Strict consensus of 10 most parsimonious trees (221 steps. Consistency index (CI) = 0.5385; retention index (RI) = 0.7198) from an unconstrained parsimony analysis. Numbers at nodes above the lines are bootstrap values greater than 50%. b–d, Phylogenetic analyses with relationships among extant taxa constrained to be compatible to a molecular-backbone tree in which extant Gnetales and Pinaceae are sister taxa (GNE-PINE tree) (Supplementary Information). b, Strict consensus of the 45 most-parsimonious trees (236 steps, CI = 0.5042, RI = 0.6786) from a constrained parsimony analysis. Numbers at nodes above the lines are bootstrap values greater than 50%. c, Maximum likelihood tree inferred from a constrained analysis. Numbers at nodes above the lines are bootstrap values. d, The 50% majority-rule consensus from a constrained Bayesian analysis (Supplementary Data 2) with posterior probabilities indicated by numbers at the nodes. BEG is the abbreviation for the Bennettitales–Erdtmanithecales–Gnetales group30. See Supplementary Information for details of characters, taxa, additional information, analyses and discussion.
Extended Data Fig. 10 Phylogenetic relationships of seed plants based on constrained parsimony.
Analyses are of the same dataset as the analyses in Extended Data Fig. 9, except for character 60: ovule-enclosing structures. The relationships among extant taxa are constrained to be compatible to a GNE-PINE molecular-backbone tree (Supplementary Information). Both trees are rooted with Elkinsia. Numbers at nodes above the lines are values of majority-rule consensus. a, The 50% majority-rule consensus of the 105 most-parsimonious trees (236 steps, CI = 0.5042, RI = 0.6759) from an analysis of the dataset in which the cupules of Triassic corystosperms, Doylea and the Inner Mongolia cupules are scored as ? for character 60. b, The 50% majority-rule consensus of the 345 most-parsimonious trees (238 steps, CI = 0.5042, RI = 0.6749) from an analysis of the dataset in which the cupules of Triassic corystosperms, Doylea and the Inner Mongolia cupules are scored as 7 (a new state) for character 60. Note that in both analyses the Bennettitales–Erdtmanithecales–Gnetales (BEG) group30 (orange shading) is maintained, and Caytonia and glossopterids are resolved as the closest relatives of angiosperms, but the relationships of other angiophytes—Triassic corystosperms, Petriellales and Doylea, and the cupules from Inner Mongolia described here—are not well resolved in a but are resolved as a clade in b. See Supplementary Information for details of characters, taxa, additional information, analyses and discussion.
Supplementary information
Supplementary Information
This file contains details regarding the phylogenetic analyses.
Supplementary Data 1
Morphological matrix for phylogenetic analyses.
Supplementary Data 2
Morphological matrix with the MrBayes commands for constrained Bayesian analysis.
Video 1
Reconstructed slice data for specimen PB23667 showing longitudinal sections of a cupule containing two seeds.
Video 2
Three-dimensional reconstruction of a seed cone from segmented Micro-CT data for specimen PB23672.
Rights and permissions
About this article
Cite this article
Shi, G., Herrera, F., Herendeen, P.S. et al. Mesozoic cupules and the origin of the angiosperm second integument. Nature 594, 223–226 (2021). https://doi.org/10.1038/s41586-021-03598-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03598-w
This article is cited by
-
Plastid phylogenomics and fossil evidence provide new insights into the evolutionary complexity of the ‘woody clade’ in Saxifragales
BMC Plant Biology (2024)
-
Intra-gastric phytoliths provide evidence for folivory in basal avialans of the Early Cretaceous Jehol Biota
Nature Communications (2023)
-
Case not closed: the mystery of the origin of the carpel
EvoDevo (2021)
-
Ancient seeds spill secrets about the evolution of flowering plants
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.