Abstract
In comets, iron and nickel are found in refractory dust particles or in metallic and sulfide grains1. So far, no iron- or nickel-bearing molecules have been observed in the gaseous coma of comets2. Iron and a few other heavy atoms, such as copper and cobalt, have been observed only in two exceptional objects: the Great Comet of 18823 and, almost a century later, C/1965 S1 (Ikeya–Seki)4,5,6,7,8,9. These sungrazing comets approached the Sun so closely that refractory materials sublimated, and their relative abundance of nickel to iron was similar to that of the Sun and meteorites7. More recently, the presence of iron vapour was inferred from the properties of a faint tail in comet C/2006 P1 (McNaught) at perihelion10, but neither iron nor nickel was reported in the gaseous coma of comet 67P/Churyumov–Gerasimenko by the in situ Rosetta mission11. Here we report that neutral Fe i and Ni i emission lines are ubiquitous in cometary atmospheres, even far from the Sun, as revealed by high-resolution ultraviolet–optical spectra of a large sample of comets of various compositions and dynamical origins. The abundances of both species appear to be of the same order of magnitude, contrasting the typical Solar System abundance ratio.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets analysed during the current study are available at the ESO Science Archive Facility at http://archive.eso.org/eso/eso_archive_main.html, under programme numbers 073.C-0525, 075.C- 0355(A), 080.C-0615, 086.C-0958, 087.C-0929, 270.C-5043, 274.C-5015, 2100.C-5035(A), 280.C-5053 and 2101.C-5051.
References
Zolensky, M. E. et al. Mineralogy and petrology of comet 81P/Wild 2 nucleus samples. Science 314, 1735–1739 (2006).
Bockelée-Morvan, D. & Biver, N. The composition of cometary ices. Philos. Trans. R. Soc. Lond. A 375, 20160252 (2017).
Copeland, R. & Lohse, J. G. in Copernicus: an International Journal of Astronomy Vol. 2, 225–244 (1882).
Arpigny, C. in Liege International Astrophysical Colloquia Vol. 22 (eds Boury, A. et al.) 189–197 (1979).
Combi, M. R., DiSanti, M. A. & Fink, U. The spatial distribution of gaseous atomic sodium in the comae of comets: evidence for direct nucleus and extended plasma sources. Icarus 130, 336–354 (1997).
Dufay, J., Swings, P. & Fehrenbach, Ch. Spectrographic observations of comet Ikeya-Seki (1965f). Astrophys. J. 142, 1698 (1965).
Preston, G. W. The spectrum of Ikeya-Seki (1965f). Astrophys. J. 147, 718–742 (1967).
Slaughter, C. D. The emission spectrum of comet Ikeya-Seki 1965-f at perihelion passage. Astron. J. 74, 929 (1969).
Thackeray, A. D., Feast, M. W. & Warner, B. Daytime spectra of comet Ikeya-Seki near perihelion. Astrophys. J. 143, 276 (1966).
Fulle, M. et al. Discovery of the atomic iron tail of comet McNaught using the heliospheric imager on STEREO. Astrophys. J. Lett. 661, L93–L96 (2007).
Altwegg, K. & ROSINA Team. Chemical highlights from the Rosetta mission. In Proceedings of the International Astronomical Union Vol. 13, 153–162 (IAU, 2017).
Arpigny, C. et al. Anomalous nitrogen isotope ratio in comets. Science 301, 1522–1524 (2003).
Guzik, P. & Drahus, M. Gaseous atomic nickel in the coma of interstellar comet 2I/Borisov. Nature https://doi.org/10.1038/s41586-021-03485-4 (2021).
Arpigny, C. On the nature of comets. In Proceedings of the Robert A. Welch Foundation Conferences on Chemical Research XXI, Cosmochemistry (ed. Mulligan, W. O.) 9 (1978).
Cochran, A. L. & Schleicher, D. G. Observational constraints on the lifetime of cometary H2O. Icarus 105, 235–253 (1993).
Biver, N. et al. Long-term monitoring of the outgassing and composition of comet 67P/Churyumov–Gerasimenko with the Rosetta/MIRO instrument. Astron. Astrophys. 630, A19 (2019).
Womack, M., Sarid, G. & Wierzchos, K. CO in distantly active comets. Publ. Astron. Soc. Pacif. 129, 031001 (2017).
Lodders, K. Solar elemental abundances. In Oxford Research Encyclopedia of Planetary Science https://doi.org/10.1093/acrefore/9780190647926.013.145 (2020).
Larimer, J. W. & Anders, E. Chemical fractionations in meteorites – III. Major element fractionations in chondrites. Geochim. Cosmochim. Acta 34, 367–387 (1970).
Grossman, L. & Larimer, J. W. Early chemical history of the solar system. Rev. Geophys. Space Phys. 12, 71–101 (1974).
Lewis, J. S. Physics and Chemistry of the Solar System 2nd edn (Academic Press, 2004).
Berger, E. L., Zega, T. J., Keller, L. P. & Lauretta, D. S. Evidence for aqueous activity on comet 81P/Wild 2 from sulfide mineral assemblages in Stardust samples and CI chondrites. Geochim. Cosmochim. Acta 75, 3501–3513 (2011).
Bradley, J. P. Chemically anomalous, preaccretionally irradiated grains in interplanetary dust from comets. Science 265, 925–929 (1994).
Ishii, H. A. et al. Comparison of comet 81P/Wild 2 dust with interplanetary dust from comets. Science 319, 447 (2008).
Bockelée-Morvan, D. et al. Comet 67P outbursts and quiescent coma at 1.3 au from the Sun: dust properties from Rosetta/VIRTIS-H observations. Mon. Not. R. Astron. Soc. 469, S443–S458 (2017).
Hensley, B. S. & Draine, B. T. Thermodynamics and charging of interstellar iron nanoparticles. Astrophys. J. 834, 134 (2017).
Ip, W.-H. & Jorda, L. Can the sodium tail of Comet Hale–Bopp have a dust-impact origin? Astrophys. J. 496, L47–L49 (1998).
Wurz, P. et al. Solar wind sputtering of dust on the surface of 67P/Churyumov–Gerasimenko. Astron. Astrophys. 583, A22 (2015).
Bloch, M. R. & Wirth, H. L. Abiotic organic synthesis in space. Naturwissenschaften 67, 562–564 (1980).
Klotz, A. et al. Possible contribution of organometallic species in the solar system ices. Reactivity and spectroscopy. Planet. Space Sci. 44, 957–965 (1996).
Huebner, W. F. Dust from cometary nuclei. Astron. Astrophys. 5, 286–297 (1970).
Tarakeshwar, P., Buseck, P. R. & Timmes, F. X. On the structure, magnetic properties, and infrared spectra of iron pseudocarbynes in the interstellar medium. Astrophys. J. 879, 2 (2019).
Smith, K. E., House, C. H., Arevalo, R. D., Dworkin, J. P. & Callahan, M. P. Organometallic compounds as carriers of extraterrestrial cyanide in primitive meteorites. Nat. Commun. 10, 2777 (2019).
Prialnik, D., Benkhoff, J. & Podolak, M. in Comets II (eds Festou et al.) 359–387 (Univ. of Arizona Press, 2004).
Levison, H. F. Comet taxonomy. In Completing the Inventory of the Solar System Vol. 107 (eds. Rettig, T. W. & Hahn, J. M.) 173–191 (ASP, 1996).
A’Hearn, M. F., Schleicher, D. G., Millis, R. L., Feldman, P. D. & Thompson, D. T. Comet Bowell 1980b. Astron. J. 89, 579–591 (1984).
Schleicher, D. G. The fluorescence efficiencies of the CN violet bands in comets. Astron. J. 140, 973–984 (2010).
Schleicher, D. G. & A’Hearn, M. F. The fluorescence of cometary OH. Astrophys. J. 331, 1058 (1988).
Bhardwaj, A. & Raghuram, S. A coupled chemistry-emission model for atomic oxygen green and red-doublet emissions in the comet C/1996 B2 Hyakutake. Astrophys. J. 748, 13 (2012).
Raghuram, S. et al. A physico-chemical model to study the ion density distribution in the inner coma of comet C/2016 R2(Pan-STARRS). Mon. Not. R. Astron. Soc. 501, 4035–4052 (2021).
Weaver, H., Feldman, P., A’Hearn, M., Dello Russo, N. & Stern, A. Comet 103P/Hartley. IAU Circ. 9183, 1 (2010).
Roth, N. X. et al. Probing the evolutionary history of comets: an investigation of the hypervolatiles CO, CH4, and C2H6 in the Jupiter-family Comet 21P/Giacobini–Zinner. Astron. J. 159, 42 (2020).
DiSanti, M. A. et al. Depleted carbon monoxide in fragment C of the Jupiter-family comet 73P/Schwassmann–Wachmann 3. Astrophys. J. Lett. 661, L101–L104 (2007).
Böhnhardt, H. et al. The unusual volatile composition of the Halley-type comet 8P/Tuttle: addressing the existence of an inner Oort cloud. Astrophys. J. Lett. 683, L71 (2008).
Lupu, R. E., Feldman, P. D., Weaver, H. A. & Tozzi, G.-P. The fourth positive system of carbon monoxide in the Hubble Space Telescope spectra of comets. Astrophys. J. 670, 1473–1484 (2007).
DiSanti, M. A. et al. Detection of formaldehyde emission in comet C/2002 T7 (LINEAR) at infrared wavelengths: line-by-line validation of modeled fluorescent intensities. Astrophys. J. 650, 470–483 (2006).
Paganini, L. et al. Observations of comet C/2009 P1 (Garradd) at 2.4 and 2.0 AU before perihelion. In Asteroids, Comets, Meteors 2012 Vol. 1667, 6331 (Lunar Planetary Institute, 2012).
Paganini, L. et al. The unexpectedly bright comet C/2012 F6 (Lemmon) unveiled at near-infrared wavelengths. Astron. J. 147, 15 (2013).
Biver, N. et al. The extraordinary composition of the blue comet C/2016 R2 (PanSTARRS). Astron. Astrophys. 619, A127 (2018).
Wierzchos, K. & Womack, M. C/2016 R2 (PANSTARRS): a comet rich in CO and depleted in HCN. Astron. J. 156, 34 (2018).
Faggi, S., Mumma, M. J., Villanueva, G. L., Paganini, L. & Lippi, M. Quantifying the evolution of molecular production rates of comet 21P/Giacobini–Zinner with iSHELL/NASA-IRTF. Astron. J. 158, 254 (2019).
de Val-Borro, M. et al. A survey of volatile species in Oort cloud comets C/2001 Q4 (NEAT) and C/2002 T7 (LINEAR) at millimeter wavelengths. Astron. Astrophys. 559, A48 (2013).
Yamamoto, T., Nakagawa, N. & Fukui, Y. The chemical composition and thermal history of the ice of a cometary nucleus. Astron. Astrophys. 122, 171–176 (1983).
Yamamoto, T. Formation environment of cometary nuclei in the primordial solar nebula. Astron. Astrophys. 142, 31–36 (1985).
Gilbert, A. G. & Sulzmann, K. G. P. The vapor pressure of iron pentacarbonyl. J. Electrochem. Soc. 121, 832 (1974).
Stull, D. R. Vapor pressure of pure substances. Organic and inorganic compounds. Ind. Eng. Chem. 39, 517–540 (1947).
Delsemme, A. H. Chemical composition of cometary nuclei. In IAU Colloq. 61: Comet Discoveries, Statistics, and Observational Selection (ed. Wilkening, L. L.) 85–130 (IAU, 1982).
Acknowledgements
We thank P. van Hoof for discussions on the iron atomic data and their uncertainties. We thank R. Hewins and R. Warin for discussions about various Fe- and Ni-rich compounds in meteorites. We thank C. Arpigny, D. Bockelée-Morvan, A. Decock, C. Opitom, H. Rauer, P. Rousselot and B. Yang for leading some UVES proposals, and the ESO staff for service mode observations. J.M., D.H. and E.J. are Honorary Research Director, Research Director and Senior Research Associate at the Fonds de la Recherche Scientifique (F.R.S-FNRS), respectively.
Author information
Authors and Affiliations
Contributions
J.M. analysed the spectra and the coma profiles and wrote the main text. D.H. contributed to the proposals and observations, reduced and calibrated the spectra, built the fluorescence model, computed the carbonyl sublimation properties and wrote the Supplementary Information. E.J. led the UVES proposals and made most of the observations. All authors contributed to the discussion and the final text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Dennis Bodewits and Ryan Fortenberry for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
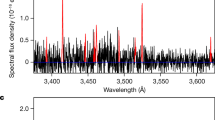
Extended Data Fig. 1 Examples of UVES comet spectra.
Comet spectra obtained with the UVES spectrograph at ESO VLT, showing many Fe i and Ni i lines in the selected wavelength region (3,425–3,530 Å). a, Spectrum of the water-poor and CO-rich long-period comet C/2016 R2 (PanSTARRS) at 3 au. b, Spectrum of the Jupiter-family comet 88P/Howell at 1.4 au. c, Spectrum of the new comet C/2002 T7 (LINEAR) at 0.68 au, with lines from the OH(1-2) band. d, Spectrum of the long-period comet C/2020 X5 (Kudo−Fujikawa). Fe i and Ni i lines are indicated by red and blue marks, respectively.
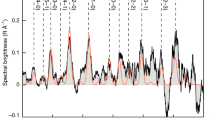
Extended Data Fig. 2 Comparisons of Fe i, Ni i and dust production rates.
a–d, The production rates of Fe i and Ni i are compared to Afρ (which is the product of the reflectivity of the grains, their filling factor and the radius of the coma; used as a proxy for the dust production rate) and to the production rates of OH, CN and CO2+, as determined from our spectra. e–f, The production rates of Fe i and Ni i are compared to those of H2O and CO measured in various previous studies for comets 8P, 9P, 21P, 73P, 103P, C/2000 WM1, C/2001 Q4, C/2002 T7, C/2009 P1, C/2012 F6 and C/2016 R2 at about the same epochs as our observations41,42,43,44,45,46,47,48,49,50,51,52. The various cometary types are colour-coded according to their dynamical classification (see Extended Data Table 1). The OH and H2O values relative to comet C/2016 R2 are upper limits. The Pearson correlation coefficients calculated without (with) the C/2016 R2 data are ρOH = 0.844 (0.531), ρAfρ = 0.644 (0.616), ρCN = 0.892 (0.518), \({\rho }_{{{{\rm{CO}}}_{2}}^{+}}\) = 0.755 (0.804), \({\rho }_{{{\rm{H}}}_{2}{\rm{O}}}\) = 0.849 (0.627) and ρCO = 0.752 (0.770).
Extended Data Fig. 3 Iron and nickel carbonyl sublimation properties.
a, Sublimation rates (Z; in molecules cm−2 s−1) of Fe and Ni carbonyls as a function of temperature, compared to those of the main ices in comets. The carbonyl rates are intermediate between those of H2O and CO2. b, The ratio of the sublimation rate of Ni(CO)4 over that of Fe(CO)5 shows that the former is considerably higher than the latter. These quantities were computed as follows. As in refs. 53,54, we estimate the condensation or sublimation temperature Ts of these compounds by solving the equation fxnkTs = Pv,x(Ts) where fx is the relative abundance of species x, n is the number density of the gas, k is the Boltzmann constant, and Pv,x is the vapour pressure, given by the relation log[Pv,x(T)] = −(A/T) + B. The constants A and B for Fe(CO)5 and Ni(CO)4 are obtained from refs. 55,56: A = 2,097 K and B = 11.62 for Fe(CO)5, A = 1,534 K and B = 10.87 for Ni(CO)4, with Pv,x in dyn cm−2. We consider relative abundances fx of 10−3–10−5 × fx(H2O) for both Fe(CO)5 and Ni(CO)4, and we adopt n = 1013 cm−3 as in ref. 54. The resulting sublimation temperatures of the iron and nickel carbonyls (97–108 K and 74–82 K, respectively, depending on fx) are between the sublimation temperatures of H2O and CO2 (152 K and 72 K), whereas CO sublimates at 25 K (ref. 54). The sublimation rate (in molecules cm−2 s−1) from the surface of a pure ice into vacuum can be expressed as57: Zx(T) = Pv,x(T)(2πmxkT)−1/2, where T is the ice temperature and mx the mass of species x.
Supplementary information
Supplementary Information
This file contains details regarding the FeI and NiI fluorescence models and abundance measurements, Supplementary Figures 1–6 and Supplementary References.
Rights and permissions
About this article
Cite this article
Manfroid, J., Hutsemékers, D. & Jehin, E. Iron and nickel atoms in cometary atmospheres even far from the Sun. Nature 593, 372–374 (2021). https://doi.org/10.1038/s41586-021-03435-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03435-0
This article is cited by
-
Cometary science with CUBES
Experimental Astronomy (2023)
-
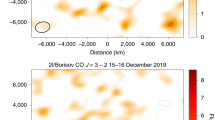
Gaseous atomic nickel in the coma of interstellar comet 2I/Borisov
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.